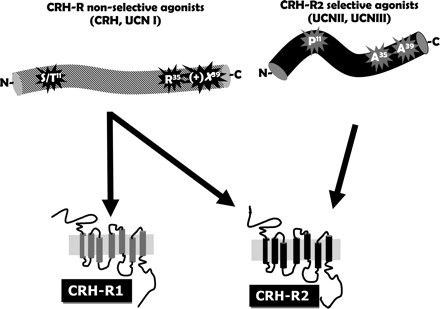
Schematic representation of amino acids within the CRH/CRH-related agonists sequence important for determining CRH-R subtype selectivity. It is now accepted that amino acid residues 32–41 are important for receptor binding, whereas residues 1–16 are responsible for both binding and receptor activation. Residues present in the domain 17–31 appear to function as a linker providing the appropriate spatial and conformational support for the two binding regions. CRH-R2 selective agonists contain a proline at position 11 and alanine residues at positions 35 and 39 (the numbering of residues is based on h/rCRH sequence). In contrast, CRH-R nonselective peptides contain an arginine at position 35 and an acidic amino acid at position 39.
Edward W. Hillhouse and Dimitris K. Grammatopoulos . Endocrine Reviews 27 (3): 260-286

Social Network Confirmation