Catalog # |
Size |
Price |
|
|---|---|---|---|
| RK-031-30 | 125 tubes | $816 |
| View/Download (PDF) - for reference only | |
|
| Suggested Procedure |
 Data may differ slightly based on lot. | 84.7 pg/ml |
|
| 10 - 1280 pg/ml |
|
| Each kit contains 1.5 µCi of Iodine 125. |
|
| This package conforms to the 49 CFR173.421 for expected radioactive material limited quantity, N.O.S. UN2910. |
|
| View/Download(PDF) |

Ghrelin and obestatin are two gastrointestinal peptides, derived from a common precursor. Expression of both peptides have been found in breast cancer tissue and ghrelin has been associated with breast cancer development. Ghrelin expression is associated with longer survival in women diagnosed with invasive and node negative breast cancer. The clinical implications of the peptide expression in male breast cancer are unclear. The aim of this study was to investigate the role and potential clinical value of ghrelin and obestatin in male breast cancer. A tissue microarray of invasive male breast cancer specimens from 197 patients was immunostained with antibodies versus the two peptides. The expression of the peptides was correlated to previously known prognostic factors in breast cancer and to the outcome. No strong correlations were found between ghrelin or obestatin expression and other known prognostic factors. Only ghrelin expression was statistically significantly correlated to breast cancer-specific survival (HR 0.39, 95% CI 0.18-0.83) in univariate analyses and in multivariate models, adjusted for tumor size and node status (HR 0.38, 95% CI 0.17-0.87). HR for obestatin was 0.38 (95% CI 0.11-1.24). Ghrelin is a potential prognostic factor for breast cancer death in male breast cancer. Patients with tumors expressing ghrelin have a 2.5-fold lower risk for breast cancer death than those lacking ghrelin expression. Drugs targeting ghrelin are currently being investigated in clinical studies treating metabolic or nutritional disorders. Ghrelin should be further evaluated in forthcoming studies as a prognostic marker with the aim to be included in decision algorithms.
Grönberg M, Nilsson C, Markholm I, et al. Ghrelin expression is associated with a favorable outcome in male breast cancer. Sci Rep. 2018;8(1):13586.
Ghrelin and obestatin are gastrointestinal peptides, encoded by the same preproghrelin gene. Both are expressed in breast cancer tissue and ghrelin has been implicated in breast cancer tumorigenesis. Despite recent advances in breast cancer management the need for new prognostic markers and potential therapeutic targets in breast cancer remains high. We studied the prognostic impact of ghrelin and obestatin in women with node negative breast cancer. Within a cohort of women with breast cancer with tumor size ≤ 50 mm, no lymph node metastases and no initiation of adjuvant chemotherapy, 190 women were identified who died from breast cancer and randomly picked 190 women alive at the corresponding time as controls. Tumor tissues were immunostained with antibodies versus the peptides. Ghrelin expression was associated with better breast cancer specific survival in univariate analyses (OR 0.55, 95% CI 0.36-0.84) and in multivariate models, adjusted for endocrine treatment and age (OR 0.57, 95% CI 0.36-0.89). Obestatin expression was non-informative (OR 1.2, 95% CI 0.60-2.46). Ghrelin expression is independent prognostic factor for breast cancer death in node negative patients-halving the risk for dying of breast cancer. Our data implies that ghrelin could be a potential therapeutic target in breast cancer treatment.
This publication used a ghrelin antibody (#H-031-30) from Phoenix Pharmaceuticals for IHC staining of more than 190 tissues samples.
Grönberg M, Ahlin C, Naeser Y, Janson ET, Holmberg L, Fjällskog ML. Ghrelin is a prognostic marker and a potential therapeutic target in breast cancer. PLoS ONE. 2017;12(4):e0176059.
BACKGROUND: Whether plasma ghrelin/obestatin levels are associated with Helicobacter pylori (H. pylori) infection, subtypes of functional dyspepsia (FD), and gastric mucosal histology has not yet been established in elderly patients. OBJECTIVE: The aim of this study was to determine whether plasma ghrelin and obestatin levels are related to gastric mucosal histology, H. pylori infection, and FD subtypes in elderly patients with FD.
METHODS: Ninety-two patients diagnosed with FD and older than 60 years (median age 69.4; range 60-88) were included. Clinical symptoms investigated included postprandial fullness, epigastric pain, epigastric soreness, nausea, and vomiting. According to the Rome III criteria, patients diagnosed with FD were divided into two subtypes: epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS). Plasma ghrelin and obestatin levels were measured using enzyme immunoassay, and histological examination of gastric mucosa was performed. H. pylori infection was determined by histopathological examination of gastric mucosal biopsy and/or Campylobacter-like organism test.
RESULTS: In our study, plasma ghrelin levels and plasma ghrelin/obestatin (G/O) ratio were significantly lower in subjects with intestinal metaplasia compared with those without intestinal metaplasia (ghrelin, p = 0.010; G/O ratio, p = 0.012). On the other hand, there were no significant differences in plasma ghrelin and obestatin levels between H. pylori-positive and H. pylori-negative groups. (ghrelin, p = 0.130; obestatin, p = 0.888). Similarly, no significant differences were detected between the EPS and PDS groups (ghrelin, p = 0.238; obestatin, p = 0.710).
CONCLUSIONS: Patients with intestinal metaplasia, a known precursor of gastric cancer, had significantly less plasma ghrelin levels and G/O ratio than those without intestinal metaplasia.
This publication used aghrelin ELISA kit (#EK-031-30) from Phoenix Pharmaceuticals for ghrelin levels analysis in human blood.
Kim SH, Kim JW, Byun J, Jeong JB, Kim BG, Lee KL. Plasma ghrelin level and plasma ghrelin/obestatin ratio are related to intestinal metaplasia in elderly patients with functional dyspepsia. PLoS ONE. 2017;12(4):e0175231.
Despite being unable to activate the cognate ghrelin receptor (GHS-R), unacylated ghrelin (UAG) possesses a unique activity spectrum that includes promoting bone marrow adipogenesis. Since a receptor mediating this action has not been identified, we re-appraised the potential interaction of UAG with GHS-R in the regulation of bone marrow adiposity. Surprisingly, the adipogenic effects of intra-bone marrow (ibm)-infused acylated ghrelin (AG) and UAG were abolished in male GHS-R-null mice. Gas chromatography showed that isolated tibial marrow adipocytes contain the medium-chain fatty acids utilised in the acylation of UAG, including octanoic acid. Additionally, immunohistochemistry and immunogold electron microscopy revealed that tibial marrow adipocytes show prominent expression of the UAG-activating enzyme ghrelin O-acyl transferase (GOAT), which is located in the membranes of lipid trafficking vesicles and in the plasma membrane. Finally, the adipogenic effect of ibm-infused UAG was completely abolished in GOAT-KO mice. Thus, the adipogenic action of exogenous UAG in tibial marrow is dependent upon acylation by GOAT and activation of GHS-R. This suggests that UAG is subject to target cell-mediated activation - a novel mechanism for manipulating hormone activity.
This publication used a ghrelin antibody (#H-032-12) from Phoenix Pharmaceuticals for observation of IHC staining of GOAT expression in tibial bone marrow.
Hopkins AL, Nelson TA, Guschina IA, et al. Unacylated ghrelin promotes adipogenesis in rodent bone marrow via ghrelin O-acyl transferase and GHS-R1a activity: evidence for target cell-induced acylation. Sci Rep. 2017;7:45541.
Prader-Willi syndrome (PWS) is caused by a loss of paternally expressed genes in an imprinted region of chromosome 15q. Among the canonical PWS phenotypes are hyperphagic obesity, central hypogonadism, and low growth hormone (GH). Rare microdeletions in PWS patients define a 91-kb minimum critical deletion region encompassing 3 genes, including the noncoding RNA gene SNORD116. Here, we found that protein and transcript levels of nescient helix loop helix 2 (NHLH2) and the prohormone convertase PC1 (encoded by PCSK1) were reduced in PWS patient induced pluripotent stem cell-derived (iPSC-derived) neurons. Moreover, Nhlh2 and Pcsk1 expression were reduced in hypothalami of fasted Snord116 paternal knockout (Snord116p-/m+) mice. Hypothalamic Agrp and Npy remained elevated following refeeding in association with relative hyperphagia in Snord116p-/m+ mice. Nhlh2-deficient mice display growth deficiencies as adolescents and hypogonadism, hyperphagia, and obesity as adults. Nhlh2 has also been shown to promote Pcsk1 expression. Humans and mice deficient in PC1 display hyperphagic obesity, hypogonadism, decreased GH, and hypoinsulinemic diabetes due to impaired prohormone processing. Here, we found that Snord116p-/m+ mice displayed in vivo functional defects in prohormone processing of proinsulin, pro-GH-releasing hormone, and proghrelin in association with reductions in islet, hypothalamic, and stomach PC1 content. Our findings suggest that the major neuroendocrine features of PWS are due to PC1 deficiency.
This publication used a ghrelin RIA kit (#RK-031-30) for ghrelin levels analysis in human blood sample.
Burnett LC, Leduc CA, Sulsona CR, et al. Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader-Willi syndrome. J Clin Invest. 2017;127(1):293-305.
Obestatin, a 23-amino acid peptide encoded by the ghrelin gene, and the GPR39 receptor were reported to be involved in the control of mitogenesis of gastric cancer cell lines; however, the relationship between the obestatin/GPR39 system and gastric cancer progression remains unknown. In the present study, we determined the expression levels of the obestatin/GPR39 system in human gastric adenocarcinomas and explored their potential functional roles. Twenty-eight patients with gastric adenocarcinomas were retrospectively studied, and clinical data were obtained. The role of obestatin/GPR39 in gastric cancer progression was studied in vitro using the human gastric adenocarcinoma AGS cell line. Obestatin exogenous administration in these GPR39-bearing cells deregulated the expression of several hallmarks of the epithelial-mesenchymal transition (EMT) and angiogenesis. Moreover, obestatin signaling promoted phenotypic changes via GPR39, increasingly impacting on the cell morphology, proliferation, migration and invasion of these cells. In healthy human stomachs, obestatin expression was observed in the neuroendocrine cells and GPR39 expression was localized mainly in the chief cells of the oxyntic glands. In human gastric adenocarcinomas, no obestatin expression was found; however, an aberrant pattern of GPR39 expression was discovered, correlating to the dedifferentiation of the tumor. Altogether, our data strongly suggest the involvement of the obestatin/GPR39 system in the pathogenesis and/or clinical outcome of human gastric adenocarcinomas and highlight the potential usefulness of GPR39 as a prognostic marker in gastric cancer.
This publication used a ghrelin antibody (#G-031-92) from Phoenix Pharmaceuticals for their immunocytochemistry and Ki67 cells staining.
Alén BO, Leal-lópez S, Alén MO, et al. The role of the obestatin/GPR39 system in human gastric adenocarcinomas. Oncotarget. 2016;7(5):5957-71.
Ghrelin is a gut-brain peptide hormone that plays an important role in the control of energy metabolism. In the mouse, the expression of its receptor, growth hormone secretagogue receptor (GHSR), was reported to be highest in two brain regions, the arcuate nucleus (ARC) in the hypothalamus and non-preganglionic Edinger-Westphal nucleus (npEW) in the midbrain. The npEW is also the most dominant site of urocortin 1 (Ucn1) expression in the mammalian brain and the npEW-Ucn1 neurons play an important role in stress adaptation response. Despite the importance of ghrelin for regulating food intake and body weight, mice lacking ghrelin or GHSR show no, or only a modest metabolic phenotype. Because GHSR mRNA is abundantly present in the stress-sensitive npEW, we hypothesized that ghrelin is not only involved in metabolism control but also in the stress response. To test this we studied the ghrelin-KO mice, under basal non-stressed and acute stressed conditions. Under basal conditions, KO mice have higher activation of npEW-Ucn1 neurons as demonstrated by dual label Ucn-1 and Fos immunohistochemistry. After restraint stress both wild-type (WT) and KO mice revealed an increased activation of the npEW nucleus, but we found that stress recruited Ucn1-ir neurons only in WT, but not in KO mice. We also determined Ucn1 mRNA expression in stressed and non-stressed conditions in KO and WT mice by in situ hybridization and found a strong up-regulation of Ucn1 mRNA in KO than in WT mice. Moreover, KO but not WT mice revealed stress-induced Ucn1 mRNA expression. Taken together, our data provide evidence for ghrelin actions in stress response. We propose that Ucn1 neurons in the npEW are instrumental in ghrelin´s action on the animal´s stress adaptation response.
L. Xu et al., C137 Deletion of ghrelin alters the response of Edinger-Westphal nucleus to restraint stress in the mouse. Poster session presented at IBRO 2011. 8th International Brain Research Organization World Congress of Neuroscience; 2011 July 14-18; Florence, Italy.
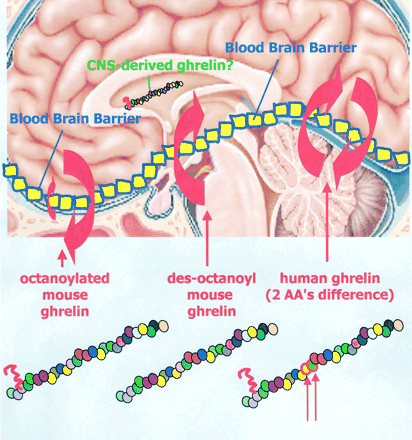
Differential transport of mouse ghrelin, des-octanoyl mouse ghrelin, and human ghrelin across the blood-brain barrier in mice. Although octanoylated (bioactive) mouse ghrelin crosses the mouse BBB predominantly in the brain-to-blood direction, passage for des-octanoyl mouse ghrelin was observed only in the blood-to-brain direction. Human ghrelin, which differs from mouse ghrelin by two amino residues only, was transported in both directions in mice. The extent and direction in which the ghrelin can cross the BBB is therefore influenced by at least two features of its primary structure, its post-translationally added fatty acid side chain and its amino acid sequence.
William A. Banks, Matthias Tschöp, Sandra M. Robinson and Mark L. Heiman. THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS. Vol. 302, Issue 2, 822-827, August 2002
We provide the first evidence for the existence in human plasma of peptides derived from the 66 carboxyl-terminal amino acids of pro-ghrelin (C-ghrelin). C-ghrelin immunoreactivity in plasma was higher than ghrelin, and did not significantly correlate with body mass index in normal health. In patients with myocardial infarction, plasma levels of both ghrelin and C-ghrelin were significantly decreased ( approximately 30%, P<0.05), whereas in patients with heart failure, C-ghrelin levels were significantly elevated ( approximately 32%, P<0.05) compared with controls. HPLC coupled with RIA showed circulating C-ghrelin to be primarily of low molecular weight (M(r) approximately 3500), but in chronic heart failure, a higher molecular weight form (M(r) approximately 7500) is also present. This is the first evidence for potential circulating hormones derived from the carboxyl terminus of pro-ghrelin and for their modulation in cardiovascular diseases.
Pemberton C, et al. Biochem Biophys Res Commun. 2003 Oct 17;310(2):567-73.
Experimental studies have suggested that ghrelin plays a role in glucose homeostasis and in the regulation of blood pressure (BP). We therefore assessed the hypothesis that a low ghrelin concentration may be a risk factor for type 2 diabetes and hypertension. We also characterized the effect of the ghrelin Arg51Gln and Leu72Met mutations on ghrelin concentrations in the population-based hypertensive (n = 519) and control (n = 526) cohorts of our OPERA (Oulu Project Elucidating Risk of Atherosclerosis) study. The fasting plasma ghrelin concentrations of 1,040 subjects were analyzed using the radioimmunoassay method. Insulin sensitivity was assessed using the quantitative insulin sensitivity check index (QUICKI). Ghrelin concentrations were negatively associated with fasting insulin (P < 0.001), systolic (P = 0.026) and diastolic BP (P = 0.018), and the prevalence of type 2 diabetes (P = 0.015) and insulin resistance (P < 0.001) in the multivariate models. In the control cohort, low ghrelin was associated with hypertension (BP >140/90 mmHg) (P = 0.031). The subjects with the ghrelin 51Gln allele had lower ghrelin concentrations than the Arg51Arg homozygotes (P = 0.001). We conclude that low ghrelin is independently associated with type 2 diabetes, insulin concentration, insulin resistance, and elevated BP. Therefore, it might have some role in the etiology of type 2 diabetes and the regulation of BP. The ghrelin Arg51Gln mutation is associated with low plasma ghrelin concentrations.
Poykko SM, et al. Diabetes. 2003 Oct;52(10):2546-53.
FIGURE 1: Effect of acute bolus administration (i.v.) of acylated ghrelin (AG: 1.0 ¦Ìg/kg) or non-acylated ghrelin (UAG; 1.0 g/kg) or AG (1.0 g/kg) + UAG (1.0 g/kg) on insulin and glucose level.
A human study investigating the biological activities of non-acylated ghrelin revealed antagonizing properties of this peptide on the hyperglycemic effects of acylated (or active) ghrelin. More specifically, bolus injections (i.v.) of this inactive form of ghrelin caused a significant reversal of the active ghrelin-induced reduction in insulin levels and ghrelin-induced increase in plasma glucose levels. This indicates a novel mechanism for the control of glucose levels in the blood and hence may lead to potential therapeutic applications of the non-acylated form of ghrelin in the treatment of type II diabetes and insulin resistance-related conditions.Broglio F, Prodam F, Benso A , et al.,Endo 2003, PHiladelphia, June 2003, Abstract #553.
Ghrelin, a growth hormone-releasing hormone produced by gastroenteropancreatic endocrine cells, hypothalamus, and pituitary, was recently identified in medullary thyroid carcinomas and derived cell lines. However, no data exist on its expression in either normal or neoplastic thyroid follicular cells. We analyzed ghrelin expression by immunohistochemistry, in situ hybridization, and reverse transcriptase-polymerase chain reaction in 15 fetal, 4 infant, and 10 adult thyroids, and in 54 tumors of follicular origin. We also analyzed the effects of ghrelin on cell proliferation in N-PAP and ARO thyroid carcinoma cell lines. Ghrelin-binding sites were investigated using reverse transcriptase-polymerase chain reaction to detect its growth hormone secretagogue receptor (GHS-R) mRNA and an in situ-binding localization procedure. Strong ghrelin immunoreactivity was found in fetal but not in infant or adult thyroids. Ghrelin protein and mRNA were present, in variable amounts, in benign and malignant tumors. Normal thyroids, thyroid tumors, and cell lines showed ghrelin binding sites by binding localization, in the absence of the specific GHS receptor mRNA (with the exception of one normal thyroid). Moreover, ghrelin induced dose-dependent inhibition of growth in cell lines. In conclusion, ghrelin is expressed in fetal but not in adult thyroid, and is re-expressed in tumors; the presence of ghrelin receptors other than GHS-R in normal and neoplastic adult thyroid is suggested; ghrelin inhibits cell proliferation of thyroid carcinoma cell lines in vitro.
Volante M., et al. American Journal of Pathology. 2003;162:645-654
Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. Ghrelin is an acyl-peptide gastric hormone acting on the pituitary and hypothalamus to stimulate growth hormone (GH) release, adiposity, and appetite. Ghrelin endocrine activities are entirely dependent on its acylation and are mediated by GH secretagogue (GHS) receptor (GHSR)-1a, a G protein-coupled receptor mostly expressed in the pituitary and hypothalamus, previously identified as the receptor for a group of synthetic molecules featuring GH secretagogue (GHS) activity. Des-acyl ghrelin, which is far more abundant than ghrelin, does not bind GHSR-1a, is devoid of any endocrine activity, and its function is currently unknown. Ghrelin, which is expressed in heart, albeit at a much lower level than in the stomach, also exerts a cardio protective effect through an unknown mechanism, independent of GH release. Here we show that both ghrelin and des-acyl ghrelin inhibit apoptosis of primary adult and H9c2 cardiomyocytes and endothelial cells in vitro through activation of extracellular signal-regulated kinase-1/2 and Akt serine kinases. In addition, ghrelin and des-acyl ghrelin recognize common high affinity binding sites on H9c2 cardiomyocytes, which do not express GHSR-1a. Finally, both MK-0677 and hexarelin, a nonpeptidyl and a peptidyl synthetic GHS, respectively, recognize the common ghrelin and des-acyl ghrelin binding sites, inhibit cell death, and activate MAPK and Akt.These findings provide the first evidence that, independent of its acylation, ghrelin gene product may act as a survival factor directly on the cardiovascular system through binding to a novel, yet to be identified receptor, which is distinct from GHSR-1a.
Baldanzi G, et al. J Cell Biol 2002 Dec 23;159(6):1029-37
![Ghrelin-[Dap] Ghrelin-[Dap]](https://phoenixpeptide.com/images/topics/ghrelin/Ghreli-dap.gif)
Measurement of circulating Ghrelin levels in fasting rat1 and human obesity2 by Phoenix's Ghrelin RIA Kit have been published in Nature and Diabetes.
Serum Ghrelin-ir in SD Rats: 1.26±0.14 ng/ml and the fasting SD Rats: 2.86±0.28 ng/ml (non-extracted)1;Plasma Ghrelin-ir in lean Caucasians: 155±25 fmol/ml and obese Caucasians: 106±23 fmol/ml2.
| Circulating Ghrelin Levels Are Decreased in Human Obesity | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader-Willi syndrome.
Burnett LC, Leduc CA, Sulsona CR, et al. J Clin Invest. 2017;127(1):293-305.
Irisin Lowers Blood Pressure by Improvement of Endothelial Dysfunction via AMPK-Akt-eNOS-NO Pathway in the Spontaneously Hypertensive Rat.
Fu J, Han Y, Wang J, et al. J Am Heart Assoc. 2016;5(11)
Hormonal responses and test meal intake among obese teenagers before and after laparoscopic adjustable gastric banding.
Sysko R, Devlin MJ, Schebendach J, et al. Am J Clin Nutr. 2013;98(5):1151-61.
Supplementation by thylakoids to a high carbohydrate meal decreases feelings of hunger, elevates CCK levels and prevents postprandial hypoglycaemia in overweight women.
Stenblom EL, Montelius C, Östbring K, et al. Appetite. 2013;68:118-23.
Adjustment of gut hormones release pattern following a fixed mealtime change in human.
Kim HH, Jeon TY, Kim YJ, et al. Clin Chim Acta. 2012;414:101-4.
Influence of acute exposure to high altitude on basal and postprandial plasma levels of gastroenteropancreatic peptides.
Riepl RL, Fischer R, Hautmann H, et al. PLoS ONE. 2012;7(9):e44445.
Effect of fasting versus feeding on the bone metabolic response to running.
Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Bone. 2012;51(6):990-9.
Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease.
Martin B, Golden E, Carlson OD, et al. Diabetes. 2009;58(2):318-28.
Obestatin promotes survival of pancreatic beta-cells and human islets and induces expression of genes involved in the regulation of beta-cell mass and function.
Granata R, Settanni F, Gallo D, et al. Diabetes. 2008;57(4):967-79.
Biological rhythm of saliva ghrelin in humans
Ayd[idot]n S, Ozercan HI, Aydin S, et al. Biological Rhythm Research. 2008; 37(2):169-177.
A comparison of leptin and ghrelin levels in plasma and saliva of young healthy subjects.
Aydin S, Halifeoglu I, Ozercan IH, et al. Peptides. 2005;26(4):647-52.
Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels.
Arosio M, Ronchi CL, Gebbia C, Cappiello V, Beck-peccoz P, Peracchi M. J Clin Endocrinol Metab. 2003;88(2):701-4.
Plasma ghrelin following cure of Helicobacter pylori.
Nwokolo CU, Freshwater DA, O'hare P, Randeva HS. Gut. 2003;52(5):637-40.
Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet.
Weigle DS, Cummings DE, Newby PD, et al. J Clin Endocrinol Metab. 2003;88(4):1577-86.
Social Network Confirmation