Catalog # |
Size |
Price |
|
|---|---|---|---|
| B-003-56 | 20 µg | $317 |
 )
)
|
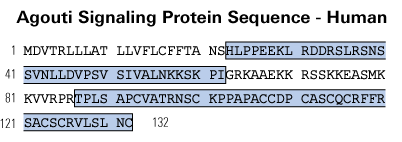
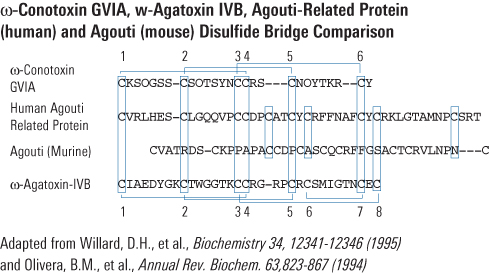
Di-Biotinyl-Lys-Ser-Ser-Arg-Arg-Cys-Val-Arg-Leu-His-Glu-Ser-Cys-Leu-Gly-Gln-Gln-Val-Pro-Cys-Cys-Asp-Pro-Cys-Ala-Thr-Cys-Tyr-Cys-Arg-Phe-Phe-Asn-Ala-Phe-Cys-Tyr-Cys-Arg-Lys-Leu-Gly-Thr-Ala-Met-Asn-Pro-Cys-Ser-Arg-Thr-NH2
|
|
Contains 5 disulfide bridges, precise positions are undetermined
|
| 6256.75 | |
|
| ≥ 90% |
|
| Exhibits correct molecular weight |
|
| Insoluble in water. Dissolve in small quantity of DMSO, followed with any desired buffer. |
|
|
Up to 6 months in lyophilized form at 0-5°C. For best results, rehydrate just before use. After rehydration, keep solution at +4°C for up to 5 days or freeze at -20°C for up to 3 months. Aliquot before freezing to avoid repeated freeze-thaw cycles. |
|
| White powder |
|
| Each vial contains 20 µg of NET labeled peptide. |
|
  |
 |
To investigate the relationship between peripheral blood levels of agouti-related protein (AGRP) and various parameters of obesity, we measured the plasma level of AGRP in 15 obese and 15 nonobese men and evaluated its relationship with body mass index (BMI), body fat weight, and visceral, sc, and total fat areas measured by computed tomography, fasting insulin levels, glucose infusion rate during an euglycemic hyperinsulinemic clamp study, serum leptin, and plasma alpha-MSH. Obese men had significantly higher plasma concentrations of AGRP than nonobese men (P < 0.01). Univariate analysis showed that the plasma levels of AGRP are proportionally correlated with BMI, body fat weight, and sc fat area in obese men (BMI: r = 0.732, P < 0.01; body fat weight: r = 0.603, P < 0.02; sc fat area: r = 0.668, P < 0.01) and in all men (BMI: r = 0.839, P < 0.0001; body fat weight: r = 0.818, P < 0.0001; sc fat area: r = 0.728, P < 0.0001). In all men, the plasma levels of AGRP were significantly correlated with the visceral fat area (r = 0.478, P < 0.01), total fat area (r = 0.655, P < 0.0001), fasting insulin level (r = 0.488, P < 0.01), glucose infusion rate (r = -0.564, P < 0.01), serum level of leptin (r = 0.661, P < 0.0001), and the plasma level of alpha-MSH (r = 0.556, P < 0.01). In all subjects, multiple regression analysis showed that the plasma levels of AGRP are significantly (F = 15.522, r = 0.801, P < 0.03) correlated with the plasma levels of alpha-MSH, independently from the total fat area. However, the correlation between plasma levels of AGRP and serum levels of leptin was found to be dependent on the total fat area. In brief, these findings showed that the circulating levels of AGRP are increased in obese men and that they are correlated with various parameters of obesity. Although correlation does not prove causation, the results of this study suggest that peripheral AGRP may play a role in the pathogenesis of obesity.
Katsuki A, Sumida Y, Gabazza EC, et al. Plasma levels of agouti-related protein are increased in obese men. J Clin Endocrinol Metab. 2001;86(5):1921-4.
Chemical synthesis of Agouti proteins - Agouti and Agouti-related proteins - is complicated by their large size and by multiple cysteine residues located in the carboxyl terminal regions. Three human Agouti-related protein (AGRP) fragments, two of which correspond to a proposed endoprotease cleavage site between amino acids 82 and 83, were synthesized and tested for anti-melanotropic activity using Xenopus laevis dermal melanophores. Amino-terminal fragments AGRP(25-51) and (54-82) were devoid of significant antagonist activity, whereas the amidated carboxyl-terminal AGRP fragment (83-132)-NH2 was potently active with an inhibitory equilibrium dissociation constant (Ki) of 0.7 nM. The ability to synthesize functionally active AGRP should help unravel its role in the central nervous system and its unusual properties with respect to interaction with the melanocortin family of G-protein coupled receptors.
Quillan JM, Sadée W, Wei ET, Jimenez C, Ji L, Chang JK. A synthetic human Agouti-related protein-(83-132)-NH2 fragment is a potent inhibitor of melanocortin receptor function. FEBS Lett. 1998;428(1-2):59-62.
Agouti-related protein (Agrp) is present in rat and human hypothalamus and is structurally related to agouti protein. Overexpression of either of these proteins results in obesity. However the effect of exogenous Agrp and its in vivo interaction with alpha-melanocyte stimulating hormone (alphaMSH), the likely endogenous melanocortin 3 and 4 receptor (MC3-R and MC4-R) agonist, have not been demonstrated. We report that 1 nmol of Agrp(83-132), a C-terminal fragment of Agrp, when administered intracerebroventricularly (ICV) into rats, increased food intake over a 24-h period (23.0+/-1.4 g saline vs 32.9+/-2.3 g Agrp, p<0.05). The hyperphagia was similar to that seen when 1 nmol of the synthetic MC3-R and MC4-R antagonist SHU9119 was given i.c.v. (19.6+/-1.8 g saline vs 32.5+/-1.7 g SHU9119, p<0.001). Both Agrp(83-132) and SHU9119 blocked the reduction in 1-h food intake of i.c.v. alphaMSH at the beginning of the dark phase. This effect occurred independently of whether the antagonists were administered simultaneously, or nine hours prior, to the alphaMSH. We have also shown Agrp(83-132) is an antagonist at the MC3-R and MC4-R, with similar inhibition of cAMP activation to that previously reported for the full length peptide. In conclusion, Agrp(83-132) administered i.c.v. increases feeding with long lasting effects and is able to inhibit the action of alphaMSH. This interaction may be mediated by the MC3-R and/or MC4-R.
Rossi M, Kim MS, Morgan DG, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139(10):4428-31.
Agouti, a gene that normally controls pigmentation in the mouse by antagonizing the melanocortin-1 receptor (MC1-R), causes obesity and diabetes when ectopically expressed1,2 , presumably by antagonism of the neural MC4-R3. Mice that lack the MC4-R gene also develop obesity, hyperinsulinaemia and hyperglycaemia4.
Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL. Overexpression of Agrt leads to obesity in transgenic mice. Nat Genet. 1997;17(3):273-4.
Expression of Agouti protein is normally limited to the skin where it affects pigmentation, but ubiquitous expression causes obesity. An expressed sequence tag was identified that encodes Agouti-related protein, whose RNA is normally expressed in the hypothalamus and whose levels were increased eightfold in ob/ob mice. Recombinant Agouti-related protein was a potent, selective antagonist of Mc3r and Mc4r, melanocortin receptor subtypes implicated in weight regulation. Ubiquitous expression of human AGRP complementary DNA in transgenic mice caused obesity without altering pigmentation. Thus, Agouti-related protein is a neuropeptide implicated in the normal control of body weight downstream of leptin signaling.
Ollmann MM, Wilson BD, Yang YK, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135-8.
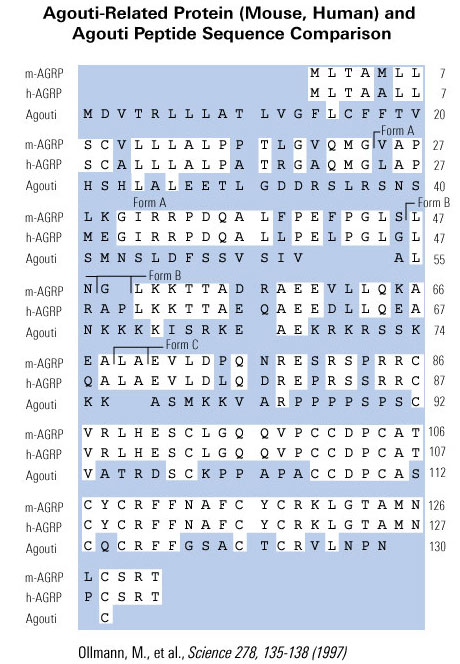
We have isolated cDNA clones that encode a novel human gene related to agouti. Sequence analysis of this gene, named ART, for agouti-related transcript, predicts a 132-amino-acid protein that is 25% identical to human agouti. The highest degree of identity is within the carboxyl terminus of both proteins. Like agouti, ART contains a putative signal sequence and a cysteine rich carboxyl terminus, but lacks the region of basic residues and polyproline residues found in the middle of the agouti protein. Both agouti and ART contain 11 cysteines, and 9 of these are conserved spatially. ART is expressed primarily in the adrenal gland, subthalamic nucleus, and hypothalamus, with a lower level of expression occurring in testis, lung, and kidney. The murine homolog of ART was also isolated and is predicted to encode a 131-amino-acid protein that shares 81% amino acid identity to humans. The mouse was found to have the same expression pattern as human when assessed by RT-PCR. Examination by in situ hybridization using mouse tissues showed localized expression in the arcuate nucleus of the hypothalamus, the median eminence, and the adrenal medulla. In addition, the hypothalamic expression of ART was elevated approximately 10-fold in ob/ob and db/db mice. ART was mapped to human chromosome 16q22 and to mouse chromosome 8D1-D2. The expression pattern and transcriptional regulation of ART, coupled with the known actions of agouti, suggests a role for ART in the regulation of melanocortin receptors within the hypothalamus and adrenal gland, and implicates this novel gene in the central control of feeding.
Shutter JR, Graham M, Kinsey AC, Scully S, Lüthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11(5):593-602.
The mRNA encoding an agouti related protein (ART) of unknown biochemical function was previously reported to be up-regulated in the hypothalamus of two genetically obese mouse strains. We have expressed human ART as a secreted protein in COS-7 cells, and show that recombinant ART is functionally active in inhibiting the binding of a radiolabeled alpha-melanocyte stimulating hormone (alpha-MSH) analog to the human melanocortin-3 (MC-3) and melanocortin-4 (MC-4) receptors, while it is not a potent inhibitor of the human melanocortin-5 (MC-5) receptor. ART is an antagonist of the human MC-3 and MC-4 receptors as determined in functional assay. ART appears to be approximately 100-fold more potent than agouti with reference to the MC-3R and MC-4R binding affinity. These data suggest that ART may be a physiological regulator of feeding behavior.
Fong TM, Mao C, Macneil T, et al. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem Biophys Res Commun. 1997;237(3):629-31.
Agouti (ASIP) and Agouti-related protein (AgRP) are endogenous antagonists of melanocortin receptors that play critical roles in the regulation of pigmentation and energy balance, respectively, and which arose from a common ancestral gene early in vertebrate evolution. The N-terminal domain of ASIP facilitates antagonism by binding to an accessory receptor, but here we show that the N-terminal domain of AgRP has the opposite effect and acts as a prodomain that negatively regulates antagonist function. Computational analysis reveals similar patterns of evolutionary constraint in the ASIP and AgRP C-terminal domains, but fundamental differences between the N-terminal domains. These studies shed light on the relationships between regulation of pigmentation and body weight, and they illustrate how evolutionary structure function analysis can reveal both unique and common mechanisms of action for paralogous gene products.
No References
| Catalog# | Product | Size | Price | Buy Now |
|---|
Social Network Confirmation