Catalog # |
Size |
Price |
|
|---|---|---|---|
| FC3-026-31 | 1 nmol | $555 |
|
| Exhibits correct molecular weight |
|
| Cy3 |
|
| 558 nm Extinction Coefficient: 130000 M-1 cm-1 |
|
| 572 nm |
|
| Each vial contains 1 nmol of NET labeled peptide. |
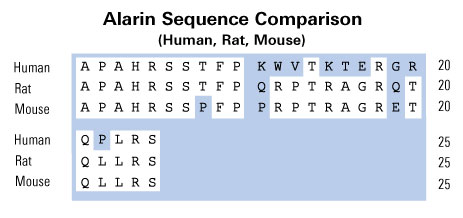
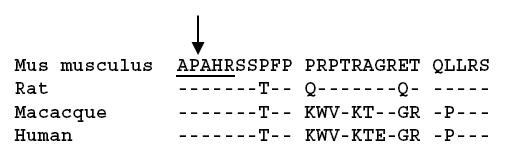
In neuroblastic tumors a relationship of differentiation of the tumor to galanin receptor expression and antiproliferative and apoptotic effects upon activation of galanin receptors in neuroblastoma cells was reported. To elucidate the expression of other components of the galanin peptide family in neuroblastic tumors, RT-PCR analysis of a variety of human neuroblastic tumor tissues was performed. Ganglioneuroma tissues revealed the presence of a splice variant of the galanin-like peptide (GALP) mRNA, which results in exclusion of exon 3 and a frame shift after the signal peptide sequence of GALP. This generates a peptide of 25 amino acids, which we have termed alarin because of the N-terminal alanine and the C-terminal serine. The novel neuropeptide alarin does not reveal significant homology to other peptides. Immunohistochemistry with antibodies directed against synthetic alarin peptide detected specific cytoplasmic granular staining in ganglia of human ganglioneuroma and ganglioneuroblastoma, as well as differentiated tumor cells of neuroblastoma tissues. Undifferentiated neuroblasts of these tumor tissues did not show alarin-like immunoreactivity and alarin-specific mRNA. Our findings indicate that alarin expression is a feature of ganglionic differentiation in neuroblastic tumor tissues.
Santic R, Fenninger K, Graf K, et al. Gangliocytes in neuroblastic tumors express alarin, a novel peptide derived by differential splicing of the galanin-like peptide gene. J Mol Neurosci. 2006;29(2):145-52.
Galanin-like peptide (GALP) is a hypothalamic neuropeptide belonging to the galanin family of peptides. The GALP gene is characterized by extensive differential splicing in a variety of murine tissues. One splice variant excludes exon 3 and results in a frame shift leading to a novel peptide sequence and a stop codon after 49 aa. In this peptide, which we termed alarin, the signal sequence of the GALP precursor peptide and the first 5 aa of the mature GALP are followed by 20 aa without homology to any other murine protein. Alarin mRNA was detected in murine brain, thymus, and skin. In accordance with its vascular localization, the peptide exhibited potent and dose-dependent vasoconstrictor and anti-edema activity in the cutaneous microvasculature, as was also observed with other members of the galanin peptide family. However, in contrast to galanin peptides in general, the physiological effects of alarin do not appear to be mediated via the known galanin receptors. Alarin adds another facet to the surprisingly high-functional redundancy of the galanin family of peptides.
Murine prepro-GALP gene splice variants. Filled boxes indicate translated peptides. Open boxes indicate 3 and 5 untranslated mRNA regions. Black boxes represent the signal peptide (SIG). Light gray squares indicate the mature GALP peptide with homology to galanin (cross-striped boxes). Dark gray boxes represent the GALP message-associated peptide (GALP-MAP), and checkered boxes represent the putative novel peptide sequences. Exon sizes are not drawn to scale. GALP(delE3) was termed alarin.
(A) Edema formation was induced by intradermal SP (300 pmol) and CGRP (10 pmol), and the effect of coinjected alarin and different galanin peptides is shown (n = 6). The response of increasing doses of alarin (1–25)
(B) and alarin (3–25)
(C) coinjected with SP plus CGRP is shown (n = 8). Results are expressed as plasma extravasation (ul/g), mean +- SEM, measured by the 125I-BSA method. Responses that are significantly different from the corresponding SP plus CGRP-treated sites are indicated
(*, P < 0.05; **, P < 0.01).
Santic R, Schmidhuber SM, Lang R, et al. Alarin is a vasoactive peptide. Proc Natl Acad Sci USA. 2007;104(24):10217-22.
No References
| Catalog# | Product | Size | Price | Buy Now |
|---|
Social Network Confirmation