Catalog # |
Size |
Price |
|
|---|---|---|---|
| MB-034-99T | 1 mL | $796 |
|
| Capture total C-peptides ( both Cα-peptide & C-peptide ) from samples for Mass-spectrometry validation & Immunoprecipitation |
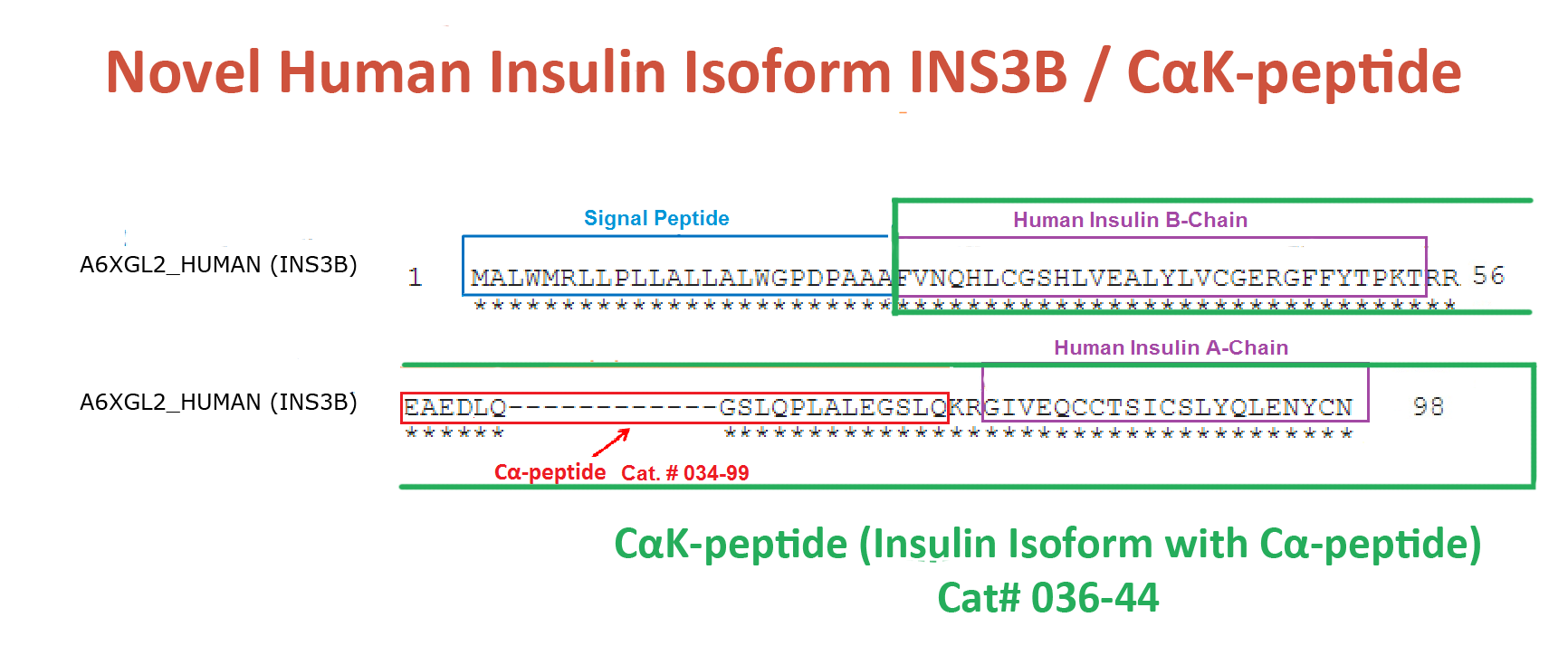

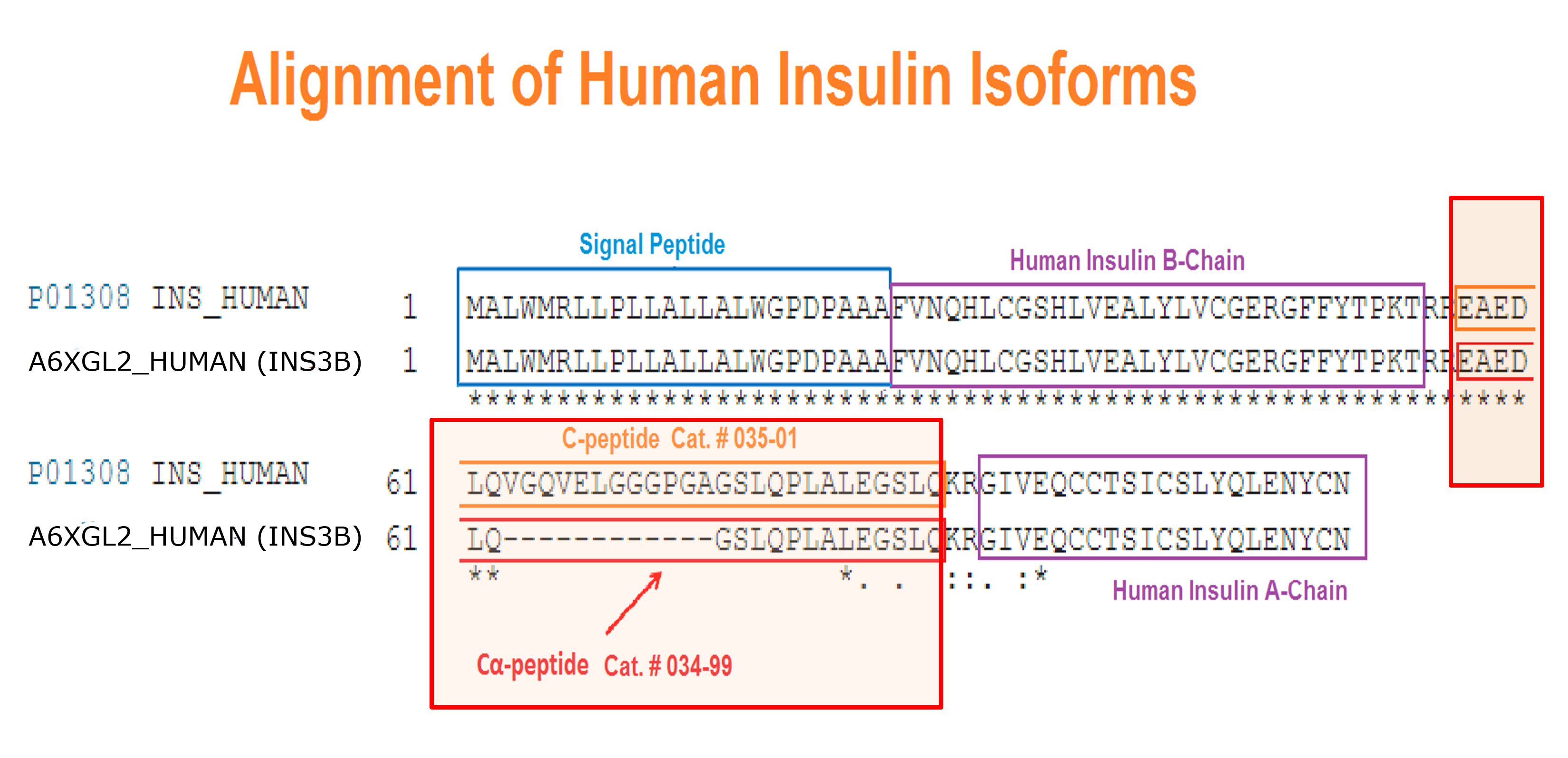
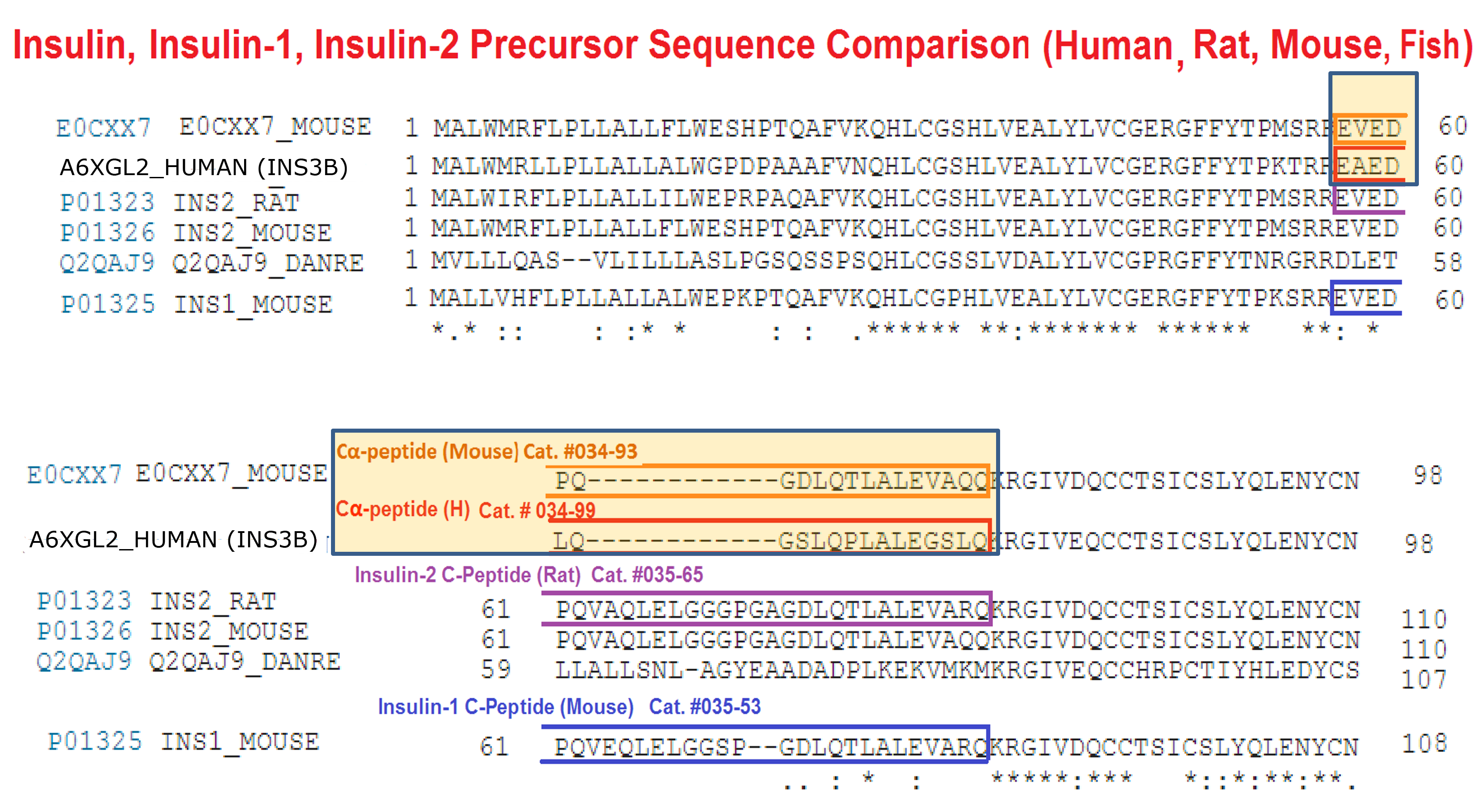
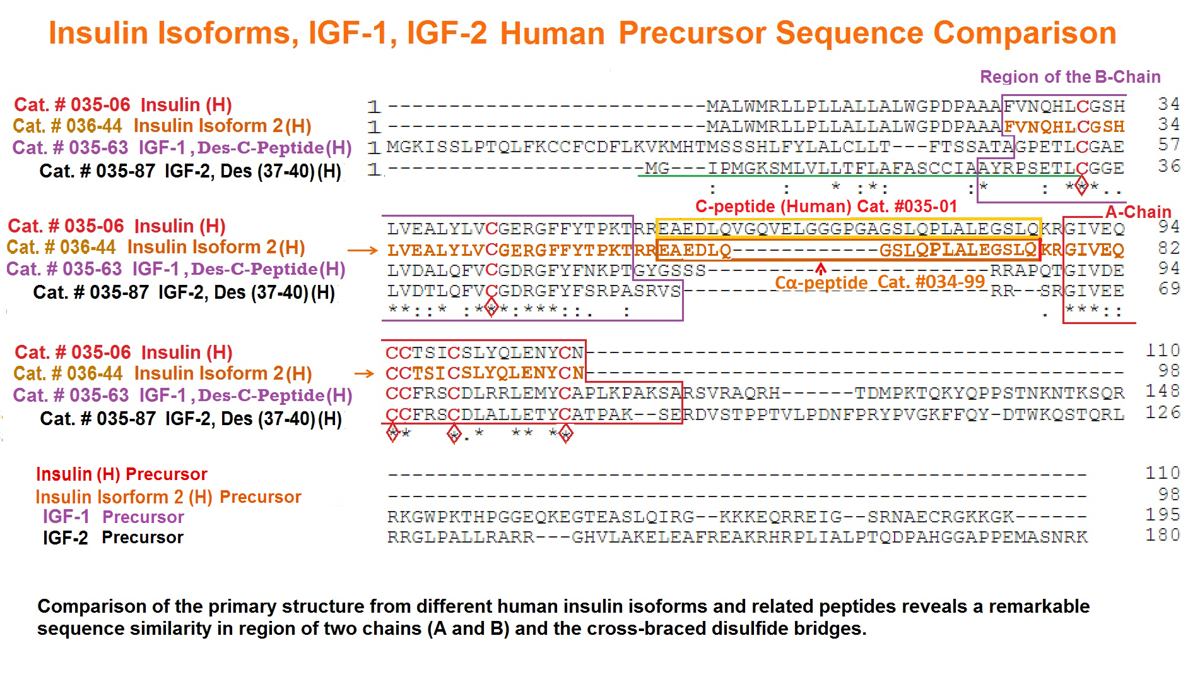
Abstract: Human insulin (INS) gene diverged from the ancestral genes of invertebrate and mammalian species millions of years ago. We previously found that mouse insulin gene (Ins2) isoforms are expressed in brain choroid plexus (ChP) epithelium cells where insulin secretion is regulated by serotonin and not by glucose. We further compared human INS isoform expression in postmortem ChP and islets of Langerhans. We uncovered novel INS upstream open reading frame (uORF) isoforms and their protein products. In addition, we found a novel alternatively spliced isoform that translates to a 74-amino acid (AA) proinsulin containing a shorter 19-AA C-peptide sequence, herein designated Cα-peptide. The middle portion of the conventional C-peptide contains β-sheet (GQVEL) and hairpin (GGGPG) motifs that are not present in Cα-peptide. Islet amyloid polypeptide (IAPP) is not expressed in ChP and its amyloid formation was inhibited in vitro by Cα-peptide more efficiently than by C-peptide. Of clinical relevance, the ratio of the 74-AA proinsulin to proconvertase processed Cα-peptide was significantly increased in islets from type 2 diabetes mellitus (T2DM) autopsy donors. Intriguingly, 100 years after the discovery of insulin we found that INS isoforms are present in ChP from insulin-deficient autopsy donors.
Liu QR, Zhu M, Zhang P, et al. Novel human insulin isoforms and cα-peptide product in islets of langerhans and choroid plexus. Diabetes. Published online October 14, 2021:db210198.
No References
| Catalog# | Product | Size | Price | Buy Now |
|---|
Social Network Confirmation