Catalog # |
Size |
Price |
|
|---|---|---|---|
| 003-98 | 5 mg | $102 |
 )
)
|
C9H14N4O5
|
| 258.2 | |
|
| ≥ 99% |
|
| Exhibits correct molecular weight |
|
| Soluble in water (10 mg/ml). |
|
|
Up to 6 months in lyophilized form at 0-5°C. For best results, rehydrate just before use. After rehydration, keep solution at +4°C for up to 5 days or freeze at -20°C for up to 3 months. Aliquot before freezing to avoid repeated freeze-thaw cycles. |
|
| White powder |
|
| Each vial contains 5 mg of material. |
|
| View/Download(PDF) |
Energy homeostasis and feeding are regulated by the central nervous system (CNS). C75, a fatty acid synthase (FAS) inhibitor, causes weight loss and anorexia, implying a novel CNS pathway(s) for sensing energy balance. AMP-activated protein kinase (AMPK), a sensor of peripheral energy balance, is phosphorylated and activated when energy sources are low. Here, we identify a role for hypothalamic AMPK in the regulation of feeding behavior and in mediating C75’s anorexic effects. AICAR, an activator of AMPK, increased food intake, whereas compound C, an inhibitor of AMPK, decreased food intake. C75 rapidly reduced the level of the phosphorylated AMPK a subunit (pAMPKα) in the hypothalamus, even in fasted mice that had elevated hypothalamic pAMPKa levels. Furthermore, AICAR reversed both the C75-induced anorexia and the decrease in hypothalamic pAMPKα levels. C75 elevated hypothalamic neuronal ATP levels, which may contribute to the mechanism by which C75 decreased AMPK activity. C75 reduced the levels of pAMPKα and phosphorylated cAMP response element binding protein (pCREB) in the arcuate nucleus neurons of the hypothalamus, suggesting a mechanism for the reduction in NPY expression seen with C75 treatment. These data indicate that modulation of FAS activity in the hypothalamus can alter energy perception via AMPK, which functions as a physiological energy sensor in the hypothalamus.
Kim EK, Miller I, Aja S, et al. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem. 2004;279(19):19970-6.
AMP-activated protein kinase (AMPK) is the downstream component of a protein kinase cascade that acts as an intracellular energy sensor maintaining the energy balance within the cell. The finding that leptin and adiponectin activate AMPK to alter metabolic pathways in muscle and liver provides direct evidence for this role in peripheral tissues. The hypothalamus is a key regulator of food intake and energy balance, coordinating body adiposity and nutritional state in response to peripheral hormones, such as leptin, peptide YY(3-36) (PYY) and ghrelin. To date the hormonal regulation of AMPK in the hypothalamus, or its potential role in the control of food intake, have not been reported. Here we demonstrate that counter-regulatory hormones involved in appetite control regulate AMPK activity, and that pharmacological activation of AMPK in the hypothalamus increases food intake. In vivo administration of leptin, which leads to a reduction in food intake, decreases hypothalamic AMPK activity. By contrast, injection of ghrelin in vivo, which increases food intake, stimulates AMPK activity in the hypothalamus. Consistent with the effect of ghrelin, injection of 5-amino-4-imidazole carboxamide (AICA) riboside, a pharmacological activator of AMPK, into either the third cerebral ventricle or directly into the paraventricular nucleus of the hypothalamus significantly increased food intake. These results suggest that AMPK is regulated in the hypothalamus by hormones which regulate food intake. Furthermore, direct pharmacological activation of AMPK in the hypothalamus is sufficient to increase food intake. These findings demonstrate that AMPK plays a role in the regulation of feeding and identify AMPK as a novel target for anti-obesity drugs.
Andersson U, Filipsson K, Abbott CR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279(13):12005-8.
Adenosine 5'-monophosphate-activated protein kinase (AMPK) now appears to be a metabolic master switch, phosphorylating key target proteins that control flux through metabolic pathways of hepatic ketogenesis, cholesterol synthesis, lipogenesis, and triglyceride synthesis, adipocyte lipolysis, and skeletal muscle fatty acid oxidation. Recent evidence also implicates AMPK as being responsible for mediating the stimulation of glucose uptake induced by muscle contraction. In addition, the secretion of insulin by insulin secreting (INS-1) cells in culture is modulated by AMPK activation. The net effect of AMPK activation is stimulation of hepatic fatty acid oxidation and ketogenesis, inhibition of cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibition of adipocyte lipolysis and lipogenesis, stimulation of skeletal muscle fatty acid oxidation and muscle glucose uptake, and modulation of insulin secretion by pancreatic beta-cells. In skeletal muscle, AMPK is activated by contraction. Type 2 diabetes mellitus is likely to be a disease of numerous etiologies. However, defects or disuse (due to a sedentary lifestyle) of the AMPK signaling system would be predicted to result in many of the metabolic perturbations observed in Type 2 diabetes mellitus. Increased recruitment of the AMPK signaling system, either by exercise or pharmaceutical activators, may be effective in correcting insulin resistance in patients with forms of impaired glucose tolerance and Type 2 diabetes resulting from defects in the insulin signaling cascade.
Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277(1 Pt 1):E1-10.
|
|
AMPK (AMP-activated Protein Kinase) an intracellular energy sensor maintaining the energy balance.AMP-activated protein kinase (AMPK) is the downstream component of a protein kinase cascade that acts as an intracellular energy sensor maintaining the energy balance within the cell. The finding that leptin and adiponectin activate AMPK to alter metabolic pathways in muscle and liver provides direct evidence for this role in peripheral tissues. The hypothalamus is a key regulator of food intake and energy balance, coordinating body adiposity and nutritional state in response to peripheral hormones, such as leptin, peptide YY(3-36) (PYY) and ghrelin. To date the hormonal regulation of AMPK in the hypothalamus, or its potential role in the control of food intake, have not been reported. Here we demonstrate that counter-regulatory hormones involved in appetite control regulate AMPK activity, and that pharmacological activation of AMPK in the hypothalamus increases food intake. In vivo administration of leptin, which leads to a reduction in food intake, decreases hypothalamic AMPK activity. By contrast, injection of ghrelin in vivo, which increases food intake, stimulates AMPK activity in the hypothalamus. Consistent with the effect of ghrelin, injection of 5-amino-4-imidazole carboxamide (AICA) riboside, a pharmacological activator of AMPK, into either the third cerebral ventricle or directly into the paraventricular nucleus of the hypothalamus significantly increased food intake. These results suggest that AMPK is regulated in the hypothalamus by hormones which regulate food intake. Furthermore, direct pharmacological activation of AMPK in the hypothalamus is sufficient to increase food intake. These findings demonstrate that AMPK plays a role in the regulation of feeding and identify AMPK as a novel target for anti-obesity drugs. |
|
|
Role of AMPK in regulating energy balance at the whole-body level. Green arrows indicate positive effects, and red lines with bars indicate negative effects. In the hypothalamus, activation of AMPK in response to low glucose or leptin levels increases food intake (18, 19); references for other effects of AMPK activation can be found in recent reviews (1). FA, fatty acid. |
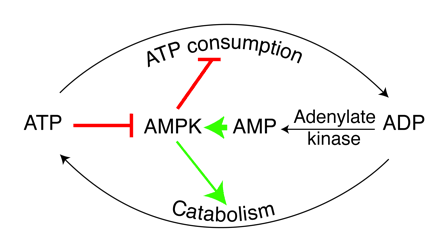 |
Role of AMPK in regulating energy balance at the single-cell level. The way in which the AMPK system controls the balance between ATP consumption (e.g., by biosynthesis, cell growth, or muscle contraction) and ATP production via catabolism is illustrated. If the rate of ATP consumption exceeds its rate of production, ADP will tend to rise and be converted to AMP by the enzyme adenylate kinase. The rise in level of the activating ligand AMP, coupled with the fall in level of the inhibitory nucleotide ATP, activates AMPK, which then switches off ATP-consuming processes and switches on catabolism in an attempt to redress the balance. |
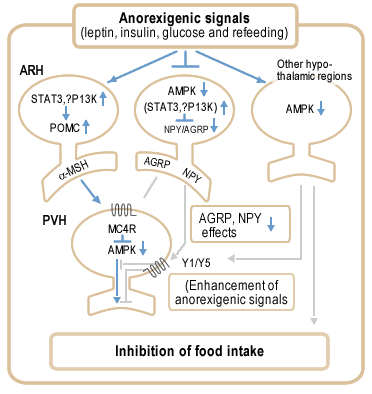 |
Proposed model for role of AMPK in anorexigenic signalling in the hypothalamus. Anorexigenic signals activate POMC neurons in ARH (arcuate hypothalamus) via STAT3 and possibly also PI3 kinase, generating a second anorexigenic signal mediated by -melanocyte stimulating hormone (-MSH). In contrast, the anorexigenic signals suppress the activity of NPY/AGRP neurons, partly via STAT3 and possibly also PI3 kinase, and decrease AMPK activity in these neurons. Decreased AMPK activity enhances the suppression of NPY/AGRP effects, leading to activation of MC4 receptor signalling in PVH (paraventricular hypothalamus) neurons. MC4 receptor activation decreases AMPK activity in PVH, which probably further enhances neurotransmission required for regulation of food intake and energy balance. Decreased NPY signalling in PVH functionally enhances the MC4 receptor signalling pathway. In addition, decreased AMPK activity in other hypothalamic regions may enhance the MC4 receptor signalling pathway by projections to the ARH or PVH (for example, NPY neurons in DMH) and recruit additional pathways that may regulate food intake. |
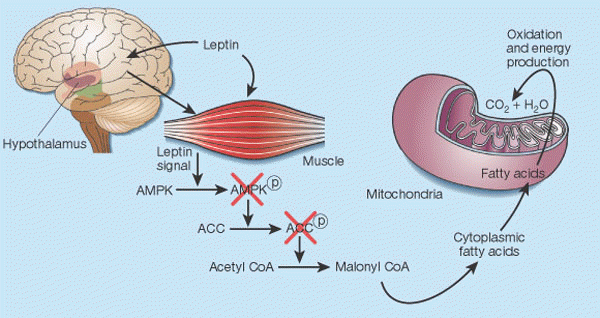 |
Leptin's control of fat in skeletal muscle2. In cells, there is a balance between transport of fatty acids into mitochondria and their subsequent oxidation, and storage of these compounds as triglycerides in the cytoplasm. This balance is regulated mainly by malonyl CoA, a fatty acid that is generated by the enzyme acetyl CoA carboxylase (ACC). Malonyl CoA inhibits transport of fatty acids into mitochondria, thereby preventing their oxidation12. Leptin causes the phosphorylation of AMP-activated protein kinase (AMPK), which in turn phosphorylates ACC, inactivating it13. Leptin thus inhibits malonyl CoA synthesis, leading to greater mitochondrial import and consumption of fatty acids. These events seem to result both from the direct action of leptin on skeletal muscle and from its indirect influence that operates through the hypothalamus. |
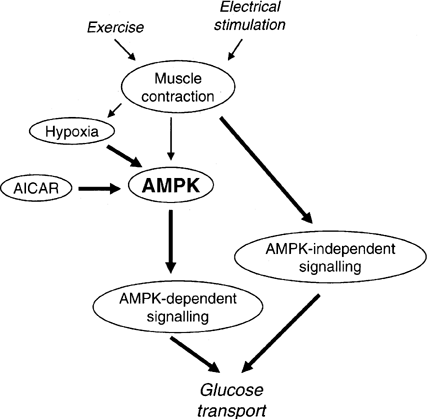 |
Figure 2 Model for the involvement of AMPK in the regulation of skeletal muscle glucose transport in response to AICAR, hypoxia, electrical stimulation and exercise. It is proposed that AICAR- and hypoxia-induced glucose uptake in skeletal muscle are AMPK-dependent, whereas exercise-induced glucose uptake is not or is only partially dependent on AMPK. It should be considered that during electrically stimulated muscle contraction (and perhaps exercise) a part of the stimulus to glucose transport may be due to hypoxia involving AMPK. Arrow thickness reflects the proposed relative involvement of the different pathways. |
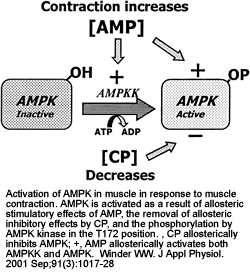 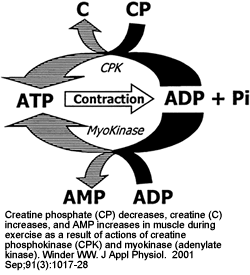 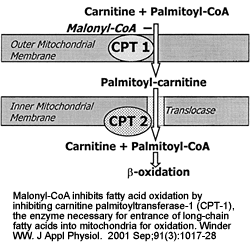 |
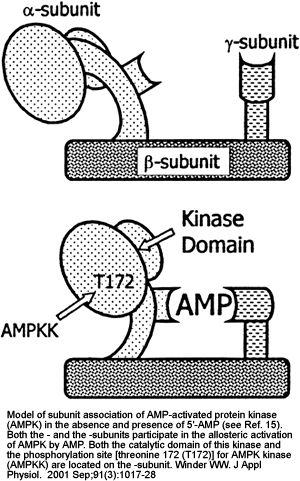 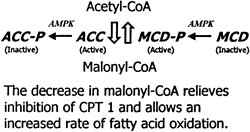 |
Malonyl-CoA content of muscle is controlled by the relative rate of synthesis by acetyl-CoA carboxylase (ACC) and the rate of degradation by malonyl-CoA decarboxylase (MCD). AMPK phosphorylates (P) and inactivates ACC. On the basis of effects of 5-aminoimidazole- 4-carboxamide -riboside (AICAR) on activation of MCD in incubated extensor digitorum longus, AMPK is hypothesized to phosphorylate and activate MCD (Ref. 80). These changes could result in a decline in malonyl-CoA (rat muscle studies). |
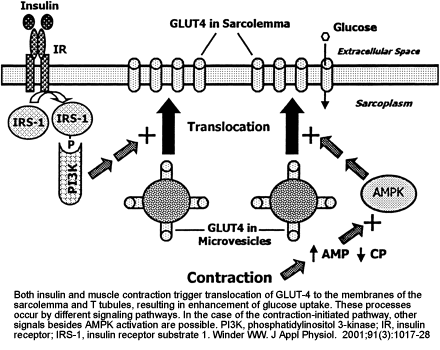
No References
| Catalog# | Product | Size | Price | Buy Now |
|---|
Social Network Confirmation