Catalog # |
Size |
Price |
|
|---|---|---|---|
| 015-70 | 100 µg | $242 |
 )
)
|
Ser-Cys-Asn-Ser-Ala-Thr-Cys-Val-Ala-His-Trp-Leu-Gly-Gly-Leu-Leu-Ser-Arg-Ala-Gly-Ser-Val-Ala-Asn-Thr-Asn-Leu-Leu-Pro-Thr-Ser-Met-Gly-Phe-Lys-Val-Tyr-NH2
|
|
Disulfide bridge(s): Cys2-Cys7
|
| 3939.53 | |
|
| ≥ 95% |
|
| Exhibits correct molecular weight |
|
| Soluble in water |
|
|
Up to 6 months in lyophilized form at 0-5°C. For best results, rehydrate just before use. After rehydration, keep solution at +4°C for up to 5 days or freeze at -20°C for up to 3 months. Aliquot before freezing to avoid repeated freeze-thaw cycles. |
|
| White powder |
|
| Each vial contains 100 µg of NET peptide. |
   |
 |
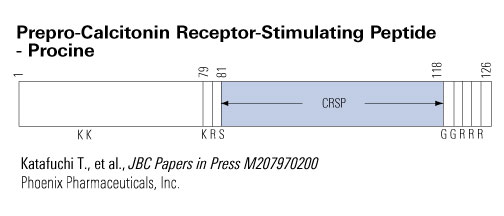
The CT-R [calcitonin (CT) receptor] is expressed in the central nervous system and is involved in the regulation of food intake, thermogenesis, and behaviors. CT-R-stimulating peptide-1 (CRSP-1), a potent ligand for the CT-R, was recently isolated from the porcine brain. In this study, we first confirmed that porcine CRSP-1 (pCRSP-1) enhanced the cAMP production in COS-7 cells expressing recombinant rat CT-R, and then we examined the central effects of pCRSP-1 on feeding and energy homeostasis in rats. Intracerebroventricular (icv) administration of pCRSP-1 to free-feeding rats suppressed food intake in a dose-dependent manner. Chronic icv infusion of pCRSP-1 suppressed body weight gain over the infusion period. Furthermore, icv administration of pCRSP-1 increased body temperature and decreased locomotor activity. The central effects of pCRSP-1 were more potent than those of porcine CT in rats. In contrast, ip administration of pCRSP-1 did not elicit any anorectic or catabolic effects. Administration icv of pCRSP-1 also induced mild dyskinesia of the lower extremities and decreased gastric acid output. Fos expression induced by icv administration of pCRSP-1 was detected in the neurons of the paraventricular nucleus, dorsomedial hypothalamic nucleus, arcuate nucleus, locus coeruleus, and nucleus of solitary tract, areas that are known to regulate feeding and energy homeostasis. Administration icv of pCRSP-1 increased plasma concentrations of ACTH and corticosterone, implying that the hypothalamic-pituitary-adrenocortical axis might be involved in catabolic effects of pCRSP-1. These results suggest that CRSP-1 can function as a ligand for the CT-R and may act as a catabolic signaling molecule in the central nervous system.
Sawada H, Yamaguchi H, Shimbara T, et al. Endocrinology. 2006;147(4):2043-50.
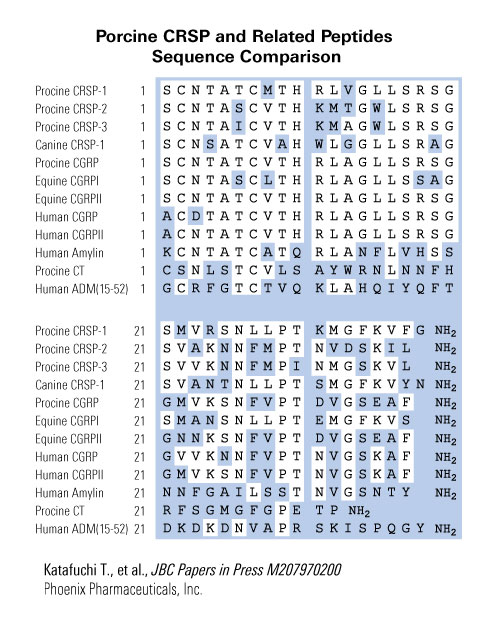
In this review, we describe the structure and biological properties of calcitonin receptor-stimulating peptide-1 (CRSP-1), CRSP-2 and CRSP-3, the novel members of the CGRP family. CRSP-1, which has been identified in the pig, cow, dog, and horse, is a specific ligand for the calcitonin (CT) receptor, and porcine CRSP-1 elicits a 100-fold greater effect on a recombinant porcine CT receptor than porcine CT, although this peptide has high structural similarity with CGRP. CRSP-1 is expressed and synthesized mainly in the central nervous system (CNS), pituitary and thyroid gland. In an in vivo experiment, bolus administration of CRSP-1 into rats reduced the plasma calcium concentration, but did not alter blood pressure, indicating its action as a CT receptor agonist in the peripheral circulation. In the CNS, CRSP-1 is also deduced to be an endogenous agonist for the CT receptor. CRSP-2 has been identified in the pig and dog, and CRSP-3 has been identified only in the pig. They are expressed and synthesized mainly in the CNS and thyroid gland. However, their endogenous molecular forms, receptors, and biological activity remain unidentified.
Katafuchi T, Minamino N. Peptides. 2004;25(11):2039-45.
We identified two cDNAs encoding new calcitonin receptor-stimulating peptides (CRSPs) in porcine hypothalamus cDNA library by cross-hybridization with the CRSP cDNA, and designated the second and third peptides as CRSP-2 and CRSP-3. The putative amino acid sequences of prepro-CRSP-2 and prepro-CRSP-3 showed higher identity with that of prepro-CRSP-1 than that of prepro-calcitonin gene-related peptide (CGRP), respectively, and these three CRSPs are considered to form a new family in the CGRP superfamily. RT-PCR analysis demonstrated that both CRSP-2 and CRSP-3 gene transcripts were expressed mainly in the central nervous system and thyroid gland. Synthetic CRSP-2 and CRSP-3 stimulated cAMP production very weakly in LLC-PK(1) cells compared with CRSP and calcitonin (CT). Furthermore, CRSP-2 and CRSP-3 did not elicit a cAMP elevation at all in the COS-7 cells expressing CT receptor or CT-like receptor with or without one of receptor activity-modifying proteins. These results suggest the presence of still unidentified action mechanisms and functions of the peptides in the CGRP superfamily.
Katafuchi T, Hamano K, Kikumoto K, Minamino N. Biochem Biophys Res Commun. 2003;308(3):445-51.
We isolated a novel biologically active peptide, designated Calcitonin Receptor-Stimulating Peptide (CRSP), from the acid extract of the porcine brain by monitoring cAMP production in the porcine kidney cell line LLC-PK1. Determination of the amino acid sequence and cDNA analysis encoding a CRSP precursor showed that this peptide has approximately 60% identity in the amino acid sequence with human calcitonin gene-related peptide type-a (aCGRP ), type-b (bCGRP ), and porcine CGRP. Northern blot analysis and radioimmunoassay demonstrated that CRSP is expressed mainly in the thyroid gland and the central nervous system, in which the calcitonin receptor was abundantly expressed. Synthetic CRSP elicited a potent stimulatory effect on the cAMP production in LLC-PK1 cells. Although it shows significant sequence similarity with CGRPs, this peptide did not elicit cAMP elevation in cells that were endogenously expressing a CGRP receptor or an adrenomedullin receptor, or were transfected with either of these recombinant receptors. Administration of CRSP into anesthetized rats did not alter the blood pressure, but induced a transient decrease in the plasma calcium concentration. In fact, this peptide potently increased the intracellular cAMP concentration in COS-7 cells that expressed the recombinant calcitonin receptor. These unique properties indicate that CRSP is not a porcine counterpart of bCGRP and probably elicits its biological effects via the calcitonin receptor.
Katafuchi T, Kikumoto K, Hamano K, Kangawa K, Matsuo H, Minamino N. J Biol Chem. 2003;278(14):12046-54.
We tested whether heterodimers comprised of calcitonin (CT) receptor lacking the 16-amino acid insert in intracellular domain 1 (CTR(I1-)) and receptor activity-modifying protein (RAMP) can function not only as calcitonin gene-related peptide (CGRP) receptors but also as adrenomedullin (AM) receptors. Whether transfected alone or together with RAMP, human (h)CTR(I1-) appeared mainly at the surface of HEK-293 cells. Expression of CTR(I1-) alone led to significant increases in cAMP in response to hCGRP or hAM, though both peptides remained about 100-fold less potent than hCT. However, the apparent potency of AM, like that of CGRP, approached that of CT when CTR(I1-) was co-expressed with RAMP. CGRP- or AM-evoked cAMP production was strongly inhibited by salmon CT-(8-32), a selective amylin receptor antagonist, but not by hCGRP-(8-37) or hAM-(22-52), antagonists of CGRP and AM receptors, respectively. Moreover, the inhibitory effects of CT-(8-32) were much stronger in cells co-expressing CTR(I1-) and RAMP than in cells expressing CTR(I1-) alone. Co-expression of CTR(I1-) with RAMP thus appears to produce functional CT-(8-32)-sensitive AM receptors.
Kuwasako K, Kitamura K, Nagoshi Y, Eto T. Biochem Biophys Res Commun. 2003;301(2):460-4.
We have recently identified in porcine brain a series of new peptides, designated calcitonin receptor-stimulating peptide-1 (CRSP-1), CRSP-2, and CRSP-3, but failed to find their counterparts in humans and rodents by either database searching or experimental cross-hybridization. In this study, we isolated cDNAs encoding precursors of bovine CRSP-1, canine CRSP-1, and canine CRSP-2 from their thyroid cDNA libraries. Although the deduced mature amino acid sequences of bovine and canine CRSP-1s and canine CRSP-2 showed identity with their respective porcine CRSP counterparts, none of them had a C-terminal amide structure. In LLC-PK(1) cells endogenously expressing the calcitonin (CT) receptor, bovine and canine CRSP-1s enhanced the cAMP production, while canine CRSP-2 did not stimulate it at all. Equine CGRP-I had a high identity in its amino acid sequence with porcine CRSP-1 and stimulated LLC-PK(1) cells at a potency comparable to that of porcine CT. None of these CRSPs or equine CGRP-I stimulated the CT-like receptor, even in the presence of receptor activity-modifying proteins. These results demonstrate that CRSP-1, a new class of biologically active peptide, is present in animals evolutionarily close to pigs and induces its activity through the calcitonin receptor, suggesting a wide existence and common properties of this peptide in mammals.
Katafuchi T, Hamano K, Minamino N. Biochem Biophys Res Commun. 2004;313(1):74-9.
No References
| Catalog# | Product | Size | Price | Buy Now |
|---|
Social Network Confirmation