Catalog # |
Size |
Price |
|
|---|---|---|---|
| 020-68 | 100 ug | $237 |
 )
)
|
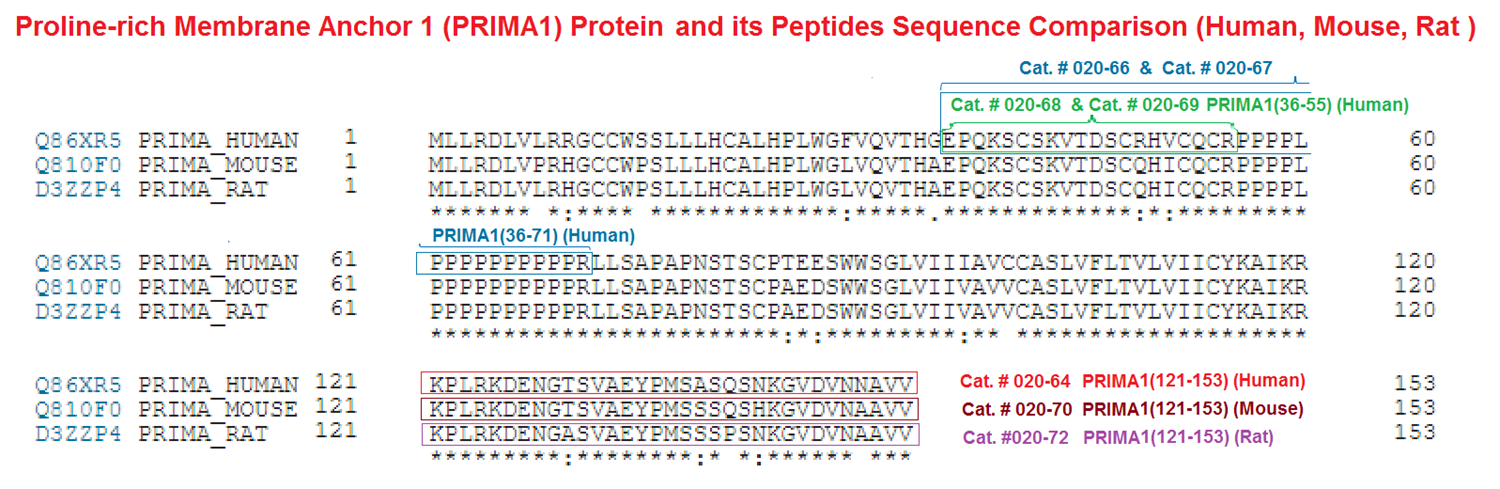
Glu-Pro-Gln-Lys-Ser-Cys-Ser-Lys-Val-Thr-Asp-Ser-Cys-Arg-His-Val-Cys-Gln-Cys-Arg
|
|
Cys1-Cys3 & Cys2-Cys4
|
| 2290.62 | |
|
| ≥ 95% |
|
| Exhibits correct molecular weight |
|
| Soluble in water |
|
|
Up to 6 months in lyophilized form at 0-5ºC. Long-term storage may form a dimerized peptide. For best results, rehydrate just before use. |
|
| White powder |
|
| Each vial contains 100 μg of NET peptide. |
Abstract: The peptidergic system is the most abundant network of ligand-receptor-mediated signaling in humans. However, the physiological roles remain elusive for numerous peptides and more than 100 G protein-coupled receptors (GPCRs). Here we report the pairing of cognate peptides and receptors. Integrating comparative genomics across 313 species and bioinformatics on all protein sequences and structures of human class A GPCRs, we identify universal characteristics that uncover additional potential peptidergic signaling systems. Using three orthogonal biochemical assays, we pair 17 proposed endogenous ligands with five orphan GPCRs that are associated with diseases, including genetic, neoplastic, nervous and reproductive system disorders. We also identify additional peptides for nine receptors with recognized ligands and pathophysiological roles. This integrated computational and multifaceted experimental approach expands the peptide-GPCR network and opens the way for studies to elucidate the roles of these signaling systems in human physiology and disease.
Foster SR, Hauser AS, Vedel L, et al. Discovery of Human Signaling Systems: Pairing Peptides to G Protein-Coupled Receptors. Cell. 2019;179(4):895-908.e21.
Abstract: G-protein-coupled receptors (GPCRs) represent an important group of targets for pharmaceutical therapeutics. The completion of the human genome revealed a large number of putative GPCRs. However, the identification of their natural ligands, and especially peptides, suffers from low discovery rates, thus impeding development of therapeutics based on these potential drug targets. We describe the discovery of novel GPCR ligands encrypted in the human proteome. Hundreds of potential peptide ligands were predicted by machine learning algorithms. In vitro screening of selected 33 peptides on a set of 152 GPCRs, including a group of designated orphan receptors, was conducted by intracellular calcium measurements and cAMP assays. The screening revealed eight novel peptides as potential agonists that specifically activated six different receptors in a dose-dependent manner. Most of the peptides showed distinct stimulatory patterns targeted at designated and orphan GPCRs. Further analysis demonstrated a significant in vivo effect for one of the peptides in a mouse inflammation model.
Shemesh R, Toporik A, Levine Z, et al. Discovery and validation of novel peptide agonists for G-protein-coupled receptors. J Biol Chem. 2008;283(50):34643-9.
Abstract: Milk of the domestic pig has 10 times more butyrylcholinesterase (BChE) per mL than porcine serum. We purified BChE from porcine milk by affinity chromatography on Hupresin-Sepharose. The pure porcine BChE (PoBChE) was a tetramer with a molecular weight of 340,000, similar to that of human BChE tetramers. The C-terminal 40 residues of PoBChE constitute the tetramerization domain. The glue that holds the 4 BChE subunits together is a polyproline-rich peptide. Mass spectrometry analysis of trypsin-digested PoBChE identified a variety of polyproline-rich peptides originating from 12 different proteins. The donor proteins exist in the nucleus or cytoplasm of cells and contribute their polyproline-rich peptides after a cell is degraded. The secreted PoBChE scavenges the polyproline-rich peptides and incorporates one polyproline peptide per PoBChE tetramer, where the polyproline peptide is bound noncovalently but very tightly with an estimated dissociation constant of 10-12 M. The most abundant polyproline-rich peptides were derived from acrosin, homeobox protein HoxB4, lysine-specific demethylase 6B, proline-rich protein 12, and proline-rich membrane anchor 1 (PRiMA). The research article associated with the data in this report can be found in Saxena et al. (2018). The Data in Brief report lists all the polyproline-rich peptides identified in PoBChE tetramers.
Abstract: GPCRs are the most successful pharmaceutical targets in history. Nevertheless, the pharmacology of many GPCRs remains inaccessible as their endogenous or exogenous modulators have not been discovered. Tools that explore the physiological functions and pharmacological potential of these 'orphan' GPCRs, whether they are endogenous and/or surrogate ligands, are therefore of paramount importance. Rates of receptor deorphanization determined by traditional reverse pharmacology methods have slowed, indicating a need for the development of more sophisticated and efficient ligand screening approaches. Here, we discuss the use of structure-based ligand discovery approaches to identify small molecule modulators for exploring the function of orphan GPCRs. These studies have been buoyed by the growing number of GPCR crystal structures solved in the past decade, providing a broad range of template structures for homology modelling of orphans. This review discusses the methods used to establish the appropriate signalling assays to test orphan receptor activity and provides current examples of structure-based methods used to identify ligands of orphan GPCRs. Linked Articles This article is part of a themed section on Molecular Pharmacology of G Protein-Coupled Receptors.
Ngo T, Kufareva I, Coleman JLj, Graham RM, Abagyan R, Smith NJ. Identifying ligands at orphan GPCRs: current status using structure-based approaches. Br J Pharmacol. 2016;173(20):2934-51.
Abstract: Acetylcholinesterase (AChE) is anchored onto cell membranes by the transmembrane protein PRiMA (proline-rich membrane anchor) as a tetrameric globular form that is prominently expressed in vertebrate brain. In parallel, the PRiMA-linked tetrameric butyrylcholinesterase (BChE) is also found in the brain. A single type of AChE-BChE hybrid tetramer was formed in cell cultures by co-transfection of cDNAs encoding AChE(T) and BChE(T) with proline-rich attachment domain-containing proteins, PRiMA I, PRiMA II, or a fragment of ColQ having a C-terminal GPI addition signal (Q(N-GPI)). Using AChE and BChE mutants, we showed that AChE-BChE hybrids linked with PRiMA or Q(N-GPI) always consist of AChE(T) and BChE(T) homodimers. The dimer formation of AChE(T) and BChE(T) depends on the catalytic domains, and the assembly of tetramers with a proline-rich attachment domain-containing protein requires the presence of C-terminal "t-peptides" in cholinesterase subunits. Our results indicate that PRiMA- or ColQ-linked cholinesterase tetramers are assembled from AChE(T) or BChE(T) homodimers. Moreover, the PRiMA-linked AChE-BChE hybrids occur naturally in chicken brain, and their expression increases during development, suggesting that they might play a role in cholinergic neurotransmission.
Chen VP, Xie HQ, Chan WK, et al. The PRiMA-linked cholinesterase tetramers are assembled from homodimers: hybrid molecules composed of acetylcholinesterase and butyrylcholinesterase dimers are up-regulated during development of chicken brain. J Biol Chem. 2010;285(35):27265-78.
No References
| Catalog# | Product | Size | Price | Buy Now |
|---|
Social Network Confirmation