Catalog # |
Size |
Price |
|
|---|---|---|---|
| 053-08 | 100 µg | $230 |
 )
)
|
Gly-Trp-Pro-Gln-Ala-Pro-Ala-Met-Asp-Gly-Ala-Gly-Lys-Thr-Gly-Ala-Glu-Glu-Ala-Gln-Pro-Pro-Glu-Gly-Lys-Gly-Ala-Arg-Glu-His-Ser-Arg-Gln-Glu-Glu-Glu-Glu-Glu-Thr-Ala-Gly-Ala-Pro-Gln-Gly-Leu-Phe-Arg-Gly-NH2
|
| 5103.45 | |
|
| ≥ 95% |
|
| Exhibits correct molecular weight |
|
| Soluble in water |
|
|
Up to 6 months in lyophilized form at 0-5°C. For best results, rehydrate just before use. After rehydration, keep solution at +4°C for up to 5 days or freeze at -20°C for up to 3 months. Aliquot before freezing to avoid repeated freeze-thaw cycles. |
|
| White powder |
|
| Each vial contains 100 µg of NET peptide. |
Pancreastatin (PST) is an endogenous peptide which regulates glucose and lipid metabolism in liver and adipose tissues. In type 2 diabetic patients, PST level is high and plays a crucial role in the negative regulation of insulin sensitivity. Novel therapeutic agents are needed to treat the diabetes and insulin resistance (IR) against the PST action. In this regard, we have investigated the PST inhibitor peptide-8 (PSTi8) action against diabetogenic PST. PSTi8 rescued PST-induced IR in HepG2 and 3T3L1 cells. PSTi8 increases the GLUT4 translocation to cell surface to promote glucose uptake in L6-GLUT4myc cells. PSTi8 treatment showed an increase in insulin sensitivity in db/db, high fat and fructose fed streptozotocin (STZ) induced IR mice. PSTi8 improved the glucose homeostasis which is comparable to metformin in diabetic mice, characterized by elevated glucose clearance, enhanced glycogenesis, enhanced glycolysis and reduced gluconeogenesis. PST and PSTi8 both were docked to the GRP78 inhibitor binding site in protein-protein docking, GRP78 expression and its ATPase activity studies. The mechanism of action of PSTi8 may be mediated by activating IRS1/2-phosphatidylinositol-3-kinase-AKT (FoxO1, Srebp-1c) signaling pathway. The discovery of PSTi8 provides a promising therapeutic agent for the treatment of metabolic diseases mainly diabetes.
Hossain Z, Valicherla GR, Gupta AP, et al. Sci Rep. 2018;8(1):8715.
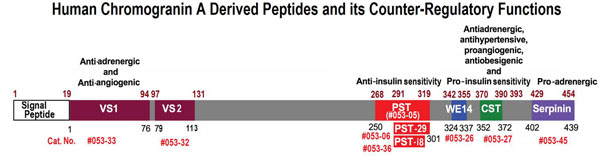
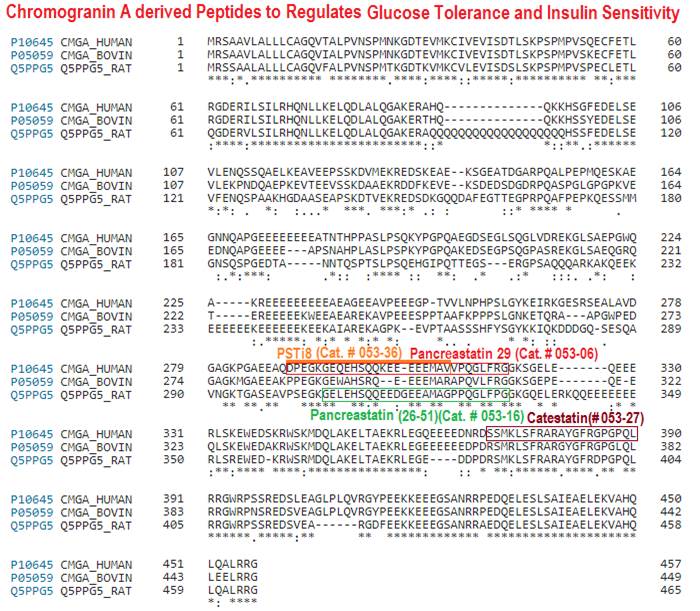
Chromogranin A (CgA) is a prohormone and granulogenic factor in endocrine and neuroendocrine tissues, as well as in neurons, and has a regulated secretory pathway. The intracellular functions of CgA include the initiation and regulation of dense-core granule biogenesis and sequestration of hormones in neuroendocrine cells. This protein is co-stored and co-released with secreted hormones. The extracellular functions of CgA include the generation of bioactive peptides, such as pancreastatin (PST), vasostatin, WE14, catestatin (CST), and serpinin. CgA knockout mice (Chga-KO) display: (i) hypertension with increased plasma catecholamines, (ii) obesity, (iii) improved hepatic insulin sensitivity, and (iv) muscle insulin resistance. These findings suggest that individual CgA-derived peptides may regulate different physiological functions. Indeed, additional studies have revealed that the pro-inflammatory PST influences insulin sensitivity and glucose tolerance, whereas CST alleviates adiposity and hypertension. This review will focus on the different metabolic roles of PST and CST peptides in insulin-sensitive and insulin-resistant models, and their potential use as therapeutic targets.
Bandyopadhyay GK, Mahata SK. Front Endocrinol (Lausanne). 2017;8:20.
BACKGROUND: Neuroendocrine tumors (NETs) of the duodenum are rare, heterogeneous, and often indolent neoplasms. We hypothesized that elevated pancreastatin levels are an indicator of a poor prognosis in well-differentiated duodenal NETs.STUDY DESIGN: Data from patients diagnosed with a primary duodenal NET were analyzed. Patients that underwent esophogogastroduodenoscopy, endoscopic ultrasound, or exploratory surgery to localize their neoplasm and whose tumors were confirmed histologically were included.RESULTS: Eighty-four patients were diagnosed with duodenal NETs from January 1991 to January 2014. Seventy-five percent and 21% of patients had their tumor localized by esophogogastroduodenoscopy and endoscopic ultrasound, respectively. The remaining 4% were localized during exploratory surgery. The 5-year Kaplan-Meier survival rate for the entire cohort (N = 84) was 80%. Survival sorted by normal vs abnormal pancreastatin level was statistically significant (p < 0.0001). Five-year survival rates were 94% and 37% for normal and abnormal pancreastatin, respectively. In contrast, survival sorted by normal vs abnormal plasma chromogranin A level was not statistically significant (p = 0.24).CONCLUSIONS: Patients with primary duodenal NETs have high 5-year survival rates. Serial monitoring of plasma pancreastatin levels can identify patients who have a poor prognosis.Woltering EA, Beyer DT, Thiagarajan R, et al. Elevated Plasma Pancreastatin, but Not Chromogranin A, Predicts Survival in Neuroendocrine Tumors of the Duodenum. J Am Coll Surg. 2016;222(4):534-42.
RATIONALE: The chromogranin A-derived peptide pancreastatin (PST) is a dysglycemic, counter-regulatory peptide for insulin action, especially in liver. Although previous evidence for a PST binding protein has been reported, such a receptor has not been identified or sequenced.
METHODS AND RESULTS: We used ligand affinity to purify the PST target, with biotinylated human PST (hCHGA273-301-amide) as "bait" and mouse liver homogenate as "prey", and identified GRP78 (a.k.a. "78 kDa Glucose Regulated Protein", HSPA5, BIP) as a major interacting partner of PST. GRP78 belongs to the family of heat shock proteins (chaperones), involved in several cellular processes including protein folding and glucose metabolism. We analyzed expression of GRP78 in the absence of PST in a mouse knockout model lacking its precursor CHGA: hepatic transcriptome data revealed global over-expression of not only GRP78 but also other heat shock transcripts (of the "adaptive UPR") in CHGA(-/-) mice compared to wild-type (+/+). By contrast, we found a global decline in expression of hepatic pro-apoptotic transcripts in CHGA(-/-) mice. GRP78's ATPase enzymatic activity was dose-dependently inhibited by PST (IC50∼5.2 µM). PST also inhibited the up-regulation of GRP78 expression during UPR activation (by tunicamycin) in hepatocytes. PST inhibited insulin-stimulated glucose uptake in adipocytes, and increased hepatic expression of G6Pase (the final step in gluconeogenesis/glycogenolysis). In hepatocytes not only PST but also other GRP78-ATPase inhibitors (VER-155008 or ADP) increased G6Pase expression. GRP78 over-expression inhibited G6Pase expression in hepatocytes, with partial restoration by GRP78-ATPase inhibitors PST, VER-155008, or ADP.
CONCLUSIONS: Our results indicate that an unexpected major hepatic target of PST is the adaptive UPR chaperone GRP78. PST not only binds to GRP78 (in pH-dependent fashion), but also inhibits GRP78's ATPase enzymatic activity, and impairs its biosynthetic response to UPR activation. PST decreases insulin-stimulated cellular glucose uptake, and PST as well as other chaperone ATPase activity inhibitors augment expression of G6Pase; GRP78 over-expression antagonizes this PST action. Analysis of the novel PST/GRP78 interaction may provide a new avenue of investigation into cellular glycemic control as well as dysglycemia.
Biswas N, Friese RS, Gayen JR, Bandyopadhyay G, Mahata SK, O'connor DT. PLoS ONE. 2014;9(1):e84132.
AIM: Acute myocardial infarction causes neurohumoral activation characterized by increased sympathetic activity. CgA is a protein released during sympathoadrenal stress from neuroendocrine tissue. Recently, increased CgA concentrations in circulation have been reported and suggested to be an independent predictor of mortality after acute myocardial infarction.MATERIALS & METHODS: Eighteen pigs underwent 1 h of regional myocardial ischemia followed by 3 h of reperfusion. Blood samples were collected every hour and plasma CgA was measured with two radioimmunoassays.RESULTS: We found a 30% increase in plasma N-terminal CgA 1 h after re-establishment of coronary blood supply. On the other hand, plasma pancreastatin did not change in response to ischemia or reperfusion but decreased during the entire experiment.CONCLUSION: Our results suggest a differentiated CgA response in myocardial reperfusion after local cardiac anoxia that may reflect tissue-specific post-translational processing and release.This publication used the porcine pancreastatin RIA kit (RK-053-08) from Phoenix Pharmaceuticals for blood level measurement.
Frydland M, Kousholt B, Larsen JR, et al. Biomark Med. 2013;7(6):959-67.
INTRODUCTION: Chromogranin A is a neuroendocrine secretory product and its loss is a feature of malignant NEN de-differentiation. We hypothesized that chromogranin A fragments were differentially expressed during NEN metastasis and played a role in the regulation of NEN proliferation.METHODS: Chromogranin A mRNA (PCR) and protein (ELISA/western blot) were studied in 10 normal human mucosa, 5 enterochromaffin cell preparations, 26 small intestinal NEN primaries and 9 liver metastases. Cell viability (WST-1 assay), proliferation (bromodeoxyuridine ELISA) and expression of AKT/AKT-P (CASE ELISA/western blot) in response to chromogranin A silencing, inhibition of prohormone convertase and mTOR inhibition (RAD001/AKT antisense) as well as different chromogranin A fragments were examined in 4 SI-NEN cell lines.RESULTS: Chromogranin A mRNA and protein levels were increased (37-340 fold, p<0.0001) in small intestinal NENs compared to normal enterochromaffin cells. Western blot identified chromogranin A-associated processing bands including vasostatin in small intestinal NENs as well as up-regulated expression of prohormone convertase in metastases. Proliferation in small intestinal NEN cell lines was decreased by silencing chromogranin A as well as by inhibition of prohormone convertase (p<0.05). This inhibition also decreased secretion of chromogranin A (p<0.05) and 5-HT (p<0.05) as well as expression of vasostatin. Metastatic small intestinal NEN cell lines were stimulated (50-80%, p<0.05) and AKT phosphorylated (Ser473: p<0.05) by vasostatin I, which was completely reversed by RAD001 (p<0.01) and AKT antisense (p<0.05) while chromostatin inhibited proliferation (~50%, p<0.05).CONCLUSION: Chromogranin A was differentially regulated in primary and metastatic small intestinal NENs and cell lines. Chromogranin A fragments regulated metastatic small intestinal NEN proliferation via the AKT pathway indicating that CgA plays a far more complex role in the biology of these tumors than previously considered.This publication used a human pancreastatin peptide from Phoenix Pharmaceuticals for peptide level evaluation.
Giovinazzo F, Schimmack S, Svejda B, et al. Chromogranin A and its fragments as regulators of small intestinal neuroendocrine neoplasm proliferation. PLoS ONE. 2013;8(11):e81111.
OBJECTIVES: Catestatin (CST) is a new endogenous neuropeptide with a potent catecholamine release-inhibitory activity. This study was to investigate plasma CST levels in patients with coronary heart disease (CHD) and to determine the clinical significance of plasma CST in cardiovascular events.METHODS: A total of 120 CHD patients and 30 age/sex-matched healthy subjects were enrolled. Plasma CST level was measured using enzyme-linked immunosorbent assay (ELISA) and norepinephrine (NE) level was measured using high-performance liquid chromatography (HPLC). Clinical and laboratory data during hospitalization were collected, and a follow-up of 1045 days was carried out.RESULTS: Compared with controls, CHD patients had significantly higher plasma CST and NE levels on admission. ST segment elevation myocardial infarction (STEMI) patients had higher CST levels than angina pectoris patients had, but CST/NE ratios were unchanged among controls and different CHD subgroups. Plasma NE was the only independent factor associated with CST. As a dichotomous variable divided by the median value, plasma CST on admission was not associated with adverse cardiovascular events.CONCLUSIONS: Plasma CST level was positively correlated with that of NE and was elevated in parallel with that of NE in the different myocardial ischemia states. Plasma CST on admission was neither associated with adverse cardiac events nor was there any significant relationship between plasma CST and onset of new cardiovascular events. The pathophysiological role of CST in CHD needs further studies.
Liu L, Ding W, Zhao F, Shi L, Pang Y, Tang C. Scand Cardiovasc J. 2013;47(4):217-24.
BACKGROUND AND OBJECTIVES: Pancreastatin is a derived peptide of chromogranin A (CgA). Pancreastatin has the potential to be a diagnostic and predictive tumor marker in detecting NETs.METHODS: Radioimmunoassay tests of pancreastatin and CgA were performed on 103 patient specimens collected at Mount Sinai Medical Center between 1/2010 and 7/2012. Patient demographics, diagnostic tests, surgical procedures, pathologic findings, adjuvant treatments, and survival were retrospectively reviewed. Statistical analysis utilized SPSS v20 software.RESULTS: Mean pancreastatin levels were significantly higher in the 92 NETs patients than in the 11 non-NETs patients (227.261 vs. 59.727, P < 0.05). Twenty-seven of the 92 patients with elevated pancreastatin levels (mean = 240.67), had normal CgA levels (mean = 4.65). Pancreastatin had sensitivity and specificity of 64% (59/92), and 100% (11/11). CgA had lower sensitivity and specificity of 43% (40/92), and 64% (7/11). In all 27 instances the pancreastatin concentration was found to be sole indicator of NET disease. When controlling for the level of CgA for the entire sample, a statistically significant difference was not found in the mean pancreastatin levels between both patient groups (P = 0.139, R = 0.484).CONCLUSION: Pancreastatin has greater sensitivity and specificity in diagnosing NETs than CgA. Further investigation of pancreastatin's diagnostic and predictive value is warranted.Rustagi S, Warner RR, Divino CM. J Surg Oncol. 2013 Aug;108(2):126-8. doi: 10.1002/jso.23359. Epub 2013 Jun 15.
Pancreastatin (PST) is processed from chromogranin A and the C-terminal amide of the peptide is an absolute requirement for biological activities. Human pancreatic carcinoma cells QGP-1 which produce both chromogranin A and PST were used to isolate cDNAs encoding two forms of peptidylglycine alpha-amidating monooxygenase (PAM). The two forms are a full length bifunctional enzyme and a variant lacking the transmembrane domain-coding region. When the cDNAs of these two forms were expressed in COS-7 cells, cells transfected with the predicted soluble form released into the culture medium a very much higher amidating activity which converts human chromogranin A-(273-302) to PST-29. The optimal pH for amidating activity was 5.4 and Cu2+, ascorbate and catalase were required as cofactors for the both forms of PAM. Km values for the membrane-bound and the soluble forms of PAM were 15.7 +/- 3.1 microM and 12.4 +/- 1.6 microM, respectively. These results demonstrate that both forms of PAM can function in the posttranslational processing of chromogranin A to PST in the environment of a secretory vesicle.
Tateishi K, Arakawa F, Misumi Y, Treston AM, Vos M, Matsuoka Y. Biochem Biophys Res Commun. 1994;205(1):282-90.
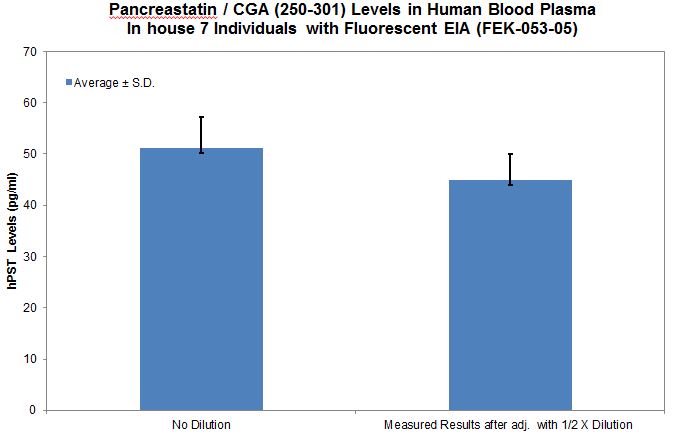
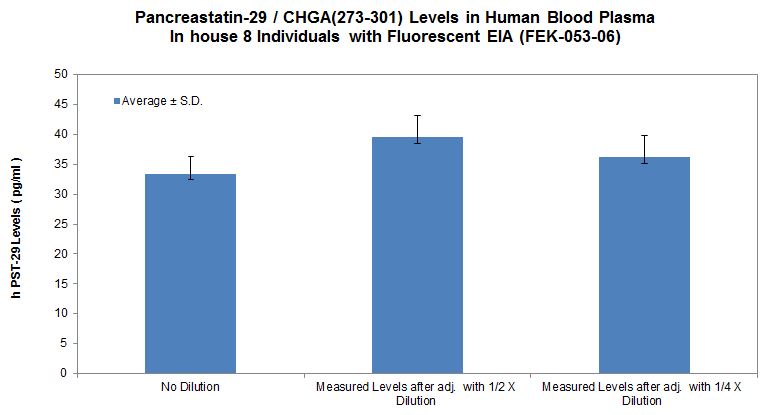
No References
| Catalog# | Product | Size | Price | Buy Now |
|---|
Social Network Confirmation