Catalog # |
Size |
Price |
|
|---|---|---|---|
| 072-66 | 100 µg | $235 |
 )
)
|
Ac-dArg-Gly-dLys-dAla-dLys-dCys-dCys-dLys-NH2
|
| 934.19 | |
|
| ≥ 95% |
|
| Exhibits correct molecular weight |
|
| Soluble in water |
|
|
Up to 6 months in lyophilized form at 0-5ºC. For best results, rehydrate just before use. After rehydration, keep solution at +4ºC for up to 5 days or freeze at -20ºC for up to 3 months. Aliquot before freezing to avoid repeated freeze-thaw cycles. |
|
| White powder |
|
| Each vial contains 100 μg of NET peptide. |
Microbial resistance against clinical used antibiotics is on the rise. Accordingly, there is a high demand for new innovative antimicrobial strategies. The host-defense peptide human beta-defensin 1 (hBD-1) is produced continuously by epithelial cells and exhibits compelling antimicrobial activity after reduction of its disulphide bridges. Here we report that proteolysis of reduced hBD-1 by gastrointestinal proteases as well as human duodenal secretions produces an eight-amino acid carboxy-terminal fragment. The generated octapeptide retains antibiotic activity, yet with distinct characteristics differing from the full-length peptide. We modified the octapeptide by stabilizing its termini and by using non-natural D-amino acids. The native and modified peptide variants showed antibiotic activity against pathogenic as well as antibiotic-resistant microorganisms, including E. coli, P. aeruginosa and C. albicans. Moreover, in an in vitro C. albicans infection model the tested peptides demonstrated effective amelioration of C. albicans infection without showing cytotoxity on human cells. In summary, protease degradation of hBD-1 provides a yet unknown mechanism to broaden antimicrobial host defense, which could be used to develop defensin-derived therapeutic applications.Wendler J, Schroeder BO, Ehmann D, et al. Proteolytic Degradation of reduced Human Beta Defensin 1 generates a Novel Antibiotic Octapeptide. Sci Rep. 2019;9(1):3640.
In the search for potential mechanisms underlying the remarkable resistance of healthy skin against infection by soil bacteria like Pseudomonas (P.) aeruginosa we identified fragments of the intrinsically disordered protein hornerin as potent microbicidal agents in the stratum corneum. We found that, independent of the amino acid (AA)-sequence, any tested linear cationic peptide containing a high percentage of disorder-promoting AA and a low percentage of order-promoting AA is a potent microbicidal antimicrobial. We further show that the antimicrobial activity of these cationic intrinsically disordered antimicrobial peptides (CIDAMPs) depends on the peptide chain length, its net charge, lipidation and environmental conditions. The ubiquitous presence of latent CIDAMP sources in nature suggests a common and yet overlooked adapted innate disinfection system of body surfaces. The simple structure and virtually any imaginable sequence or composition of disorder-promoting AA allow the generation of a plethora of CIDAMPs. These are potential novel microbicidal anti-infectives for various bacterial pathogens, including P. aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA) and fungal pathogens like Candida albicans and Cryptococcus neoformans.Latendorf T, Gerstel U, Wu Z, et al. Cationic Intrinsically Disordered Antimicrobial Peptides (CIDAMPs) Represent a New Paradigm of Innate Defense with a Potential for Novel Anti-Infectives. Sci Rep. 2019;9(1):3331.
Cationic intrinsically disordered antimicrobial peptides (CIDAMPs) belong to a novel class of epithelial peptide antibiotics with microbicidal activity against various pathogens, including Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus and Candida albicans. Here we show that treatment of distinct bacteria with different hornerin (HRNR)-derived CIDAMPs cause formation of unique cytoplasmic protein aggregates, suggesting a common intracellular mode of action. We further found that, unlike most amphipathic antimicrobial peptides, HRNR traverses bacterial membranes energy-dependently and accumulates within the cytoplasm. Strikingly, certain structurally different, HRNR-based CIDAMPs were found to bind to an identical panel of distinct bacterial ribosomal proteins, thereby manifesting features of several known classes of antibiotics. This may cause the formation of aberrant proteins and toxic protein aggregates in HRNR-treated pathogens which eventually may induce its death. Our study reveals evidence that structurally distinct CIDAMPs of an abundant body surface protein simultaneously target multiple sites of the bacterial protein synthesis machinery.Gerstel U, Latendorf T, Bartels J, Becker A, Tholey A, Schröder JM. Hornerin contains a Linked Series of Ribosome-Targeting Peptide Antibiotics. Sci Rep. 2018;8(1):16158.
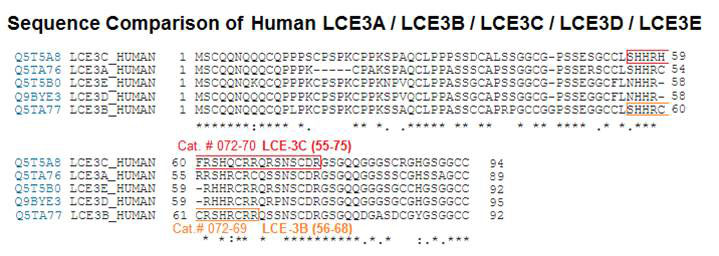
Terminally differentiating epidermal keratinocytes express a large number of structural and antimicrobial proteins that are involved in the physical barrier function of the stratum corneum and provide innate cutaneous host defense. Late cornified envelope (LCE) genes, located in the epidermal differentiation complex on chromosome 1, encode a family of 18 proteins of unknown function, whose expression is largely restricted to epidermis. Deletion of two members, LCE3B and LCE3C (LCE3B/C-del), is a widely-replicated psoriasis risk factor that interacts with the major psoriasis-psoriasis risk gene HLA-C*06. Here we performed quantitative trait locus analysis, utilizing RNA-seq data from human skin and found that LCE3B/C-del was associated with a markedly increased expression of LCE3A, a gene directly adjacent to LCE3B/C-del. We confirmed these findings in a 3-dimensional skin model using primary keratinocytes from LCE3B/C-del genotyped donors. Functional analysis revealed that LCE3 proteins, and LCE3A in particular, have defensin-like antimicrobial activity against a variety of bacterial taxa at low micromolar concentrations. No genotype-dependent effect was observed for the inside-out or outside-in physical skin barrier function. Our findings identify an unknown biological function for LCE3 proteins and suggest a role in epidermal host defense and LCE3B/C-del-mediated psoriasis risk.Niehues H, Tsoi LC, Van der krieken DA, et al. Psoriasis-Associated Late Cornified Envelope (LCE) Proteins Have Antibacterial Activity. J Invest Dermatol. 2017;137(11):2380-2388.
No References
| Catalog# | Product | Size | Price | Buy Now |
|---|
Social Network Confirmation