Around one-fifth of cancer deaths in the United States are associated with obesity. But how is fat fueling the growth of tumors? Most of the mice in Stephen Hursting’s laboratory at the University of Texas in Austin have cancer. However, among mice with identical genetics and the same tumor types, their cancers vary drastically; some have tumors bulging out of their sides, and others look healthy. The differences between the mice: their diets and the resulting amount of fat in their bodies. Hursting, a nutritional scientist, can quickly predict which mice have the most aggressive, fastest growing cancer by answering a simple question: Which mice are obese? Ample evidence exists that fat animals are more likely to develop cancer than lean animals. Cancers in fat animals also grow faster and larger, spread more quickly, and are more resistant to treatment. Furthermore, although experiments explaining the molecular basis for the phenomenon have focused on rodents and monkeys, human cancer statistics suggest that the same holds true for us: overweight and obese people get more cancer, worse cancer, and die more often from cancer than people with less body fat. In a landmark 2003 study, American Cancer Society researchers analyzed data on obesity and cancer from a group of 900,000 American adults that they had monitored for 16 years (1). The researchers found that the most obese women had a 62% increase in their risk of dying from cancer than women of normal weight; for obese men, the increase was 52%. The wide range of tumor types included colorectal, liver, gallbladder, pancreas, esophageal, kidney, prostate, breast, uterine, endometrial, and ovarian cancers. The researchers concluded that above-normal weight was associated with almost 20% of all cancer deaths in the United States. “There’s an incredibly powerful link between obesity and cancer,” says oncologist Joyce Slingerland of the University of Miami, Florida. “Everyone’s heard of obesity’s effect on heart disease and diabetes, and we’re now beginning to understand that the cancer risk is just as great,” she says. Although researchers and epidemiologists had long suspected that diet and cancer were linked, efforts to explain why being fat makes cancer more deadly have only begun to deliver results in the past decade. As obesity rates soar around the world, there are still more questions than answers. However, researchers say there are now glimmers of hope: treatments that weaken the effects of obesity on cancer in mice, and a better fundamental understanding of how fat fuels cancer.
Williams SC. Link between obesity and cancer. PNAS. 2013;110(22):8753-4.
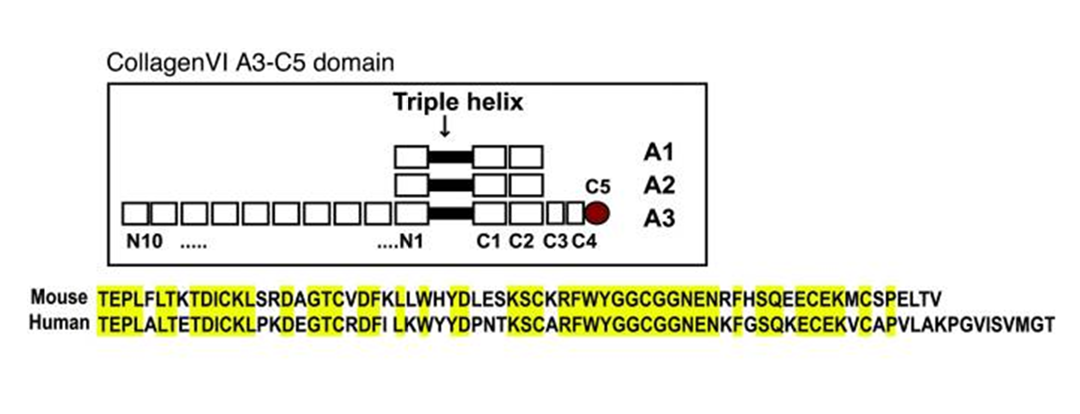
Collagen VI is a ubiquitously expressed extracellular microfibrillar protein. Its most common molecular form is composed of the α1(VI), α2(VI), and α3(VI) collagen α chains encoded by the COL6A1, COL6A2, and COL6A3 genes, respectively. Mutations in any of the three collagen VI genes cause congenital muscular dystrophy types Bethlem and Ullrich as well as intermediate phenotypes characterized by muscle weakness and connective tissue abnormalities. The α3(VI) collagen α chain has much larger N- and C-globular domains than the other two chains. Its most C-terminal domain can be cleaved off after assembly into microfibrils, and the cleavage product has been implicated in tumor angiogenesis and progression. Here we characterize a Col6a3 mutant mouse that expresses a very low level of a non-functional α3(VI) collagen chain. The mutant mice are deficient in extracellular collagen VI microfibrils and exhibit myopathic features, including decreased muscle mass and contractile force. Ultrastructurally abnormal collagen fibrils were observed in tendon, but not cornea, of the mutant mice, indicating a distinct tissue-specific effect of collagen VI on collagen I fibrillogenesis. Overall, the mice lacking normal ?3(VI) collagen chains displayed mild musculoskeletal phenotypes similar to mice deficient in the α1(VI) collagen α chain, suggesting that the cleavage product of the α3(VI) collagen does not elicit essential functions in normal growth and development. The Col6a3 mouse mutant lacking functional α3(VI) collagen chains thus serves as an animal model for COL6A3-related muscular dystrophy.
Pan TC, Zhang RZ, Markova D, et al. COL6A3 protein deficiency in mice leads to muscle and tendon defects similar to human collagen VI congenital muscular dystrophy. J Biol Chem. 2013;288(20):14320-31.
Endotrophin is a cleavage product of collagenVIα3 (COL6A3). Here, we explore the relationship between thiazolidinediones (TZDs), endotrophin and cisplatin resistance in the context of a mammary tumour model. COL6A3 levels are increased in response to cisplatin exposure in tumours. Endotrophin, in turn, causes cisplatin resistance. The effects of endotrophin can be bypassed, either through use of COL6 null (COL6(-/-)) mice or by administering TZDs in wild-type mice (leading to a downregulation of endotrophin). Both approaches sensitize tumours to cisplatin through the suppression of endotrophin-induced epithelial-mesenchymal transition. The beneficial effects of TZDs on cisplatin sensitivity are diminished in COL6(-/-) mice, whereas endotrophin(+) tumours are sensitive to the TZD/cisplatin combination. Therefore, the chemosensitization obtained with TZDs is achieved through a downregulation of endotrophin. Treatment with an endotrophin neutralizing antibody in combination with cisplatin completely inhibits tumour growth of tumour allografts. Combined, our data suggest that endotrophin levels are a strong prognostic marker for the effectiveness of the combination therapy of TZDs with cisplatin, and neutralization of endotrophin activity dramatically improves the therapeutic response to combination therapy.
Park J, Morley TS, Scherer PE. Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol Med. 2013;5(6):935-48.
ETP stimulates tumor growth and metastasis via fibrosis, angiogenesis, and inflammation.
A stromal collagen VI fragment promotes mammary tumorigenesis. American Association for Cancer Research. 2012;2(12).
Collagen VI (COL6, encoded by the COL6A1, COL6A2, and COL6A3 genes) is an extracellular matrix protein that forms a microfilamentous network in various connective tissues, including skeletal muscle, cartilage, skin and adipose tissue. Among the various tissues, adipose tissue is by far the most abundant source of COL6 microfilaments [1]. Clinically, mutations in COL6 develop mild muscle myopathies (such as Bethlem myopathy and Ullrich congenital muscular dystrophy), with symptoms of muscle weakness and apoptosis combined with joint hyperlaxity and contractures [2]. A genetically engineered mouse model, deficient in COL6 microfilament formation and secretion, has been widely used to investigate the roles of COL6 under physiological and pathological conditions. COL6 deficiency in mice leads to the development of muscle dystrophies resembling Bethlem myopathy in man [3]. In the area of tumor biology, COL6 has been identified as a tumor-promoting factor abundantly produced and released from adipocytes [4]. Subsequent analysis of the COL6 functional null mice bred into the murine MMTV-PyMT mammary tumor model (mouse mammary tumor virus-polyoma middle T antigen) showed a significant attenuation of early onset mammary tumor progression [5]. Specifically, the carboxyl-terminal domain of the COL6A3 chain is massively upregulated in the malignant tumors of human patients compared to the remaining part of COL6A3 chain [5]. We recently followed up on this phenomenon and demonstrated that the cleavage product from the carboxyl-terminus of the COL6A3 chain (that we refer to as endotrophin) accounts for the tumor-promoting effects associated with COL6 [6]. Ectopic expression of the isolated endotrophin fragment within the tumor microenvironment of MMTV-PyMT mice drives an increase of both primary tumor growth and pulmonary metastasis through an enhancement of the expansion of the tumor stroma [6]. Additional prominent effects associated with endotrophin overexpression in the tumor stroma include an increase in fibrosis, angiogenesis and inflammation through increased fibrogenesis, a stimulation of epithelial-mesenchymal transition (EMT) and chemokine activities; these are well-established stromal phenomena that support aggressive traits of tumors (Figure ?(Figure1).1). Indeed, neutralizing monoclonal antibodies against endotrophin suppress tumor growth and reduce metastatic growth in MMTV-PyMT mice [6]. EMT of tumors conveys metastatic traits and multiple drug resistances to cancer cells. Since endotrophin is a potent stimulator of EMT, it suggests that the neutralization of endotrophin may lend itself to enhance chemo-sensitivity in combination with conventional therapeutic regimens, though this remains to be directly shown.
Park J, Scherer PE. Endotrophin – Linking Obesity with Aggressive Tumor Growth. Oncotarget. 2012;3(12):1487-1488.
Adipocytes represent a major cell type in the mammary tumor microenvironment and are important for tumor growth. Collagen VI (COL6) is highly expressed in adipose tissue, upregulated in the obese state, and enriched in breast cancer lesions and is a stimulator of mammary tumor growth. Here, we have described a cleavage product of the COL6α3 chain, endotrophin (ETP), which serves as the major mediator of the COL6-mediated tumor effects. ETP augmented fibrosis, angiogenesis, and inflammation through recruitment of macrophages and endothelial cells. Moreover, ETP expression was associated with aggressive mammary tumor growth and high metastatic growth. These effects were partially mediated through enhanced TGF-β signaling, which contributes to tissue fibrosis and epithelial-mesenchymal transition (EMT) of tumor cells. Our results highlight the crucial role of ETP as an obesity-associated factor that promotes tumor growth in the context of adipocyte interactions with tumor and stromal cells.
Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest. 2012;122(11):4243-56.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 062-79 | Endotrophin (Human) | 20 µg | $444 |
| T-062-79 | Endotrophin (Human) - I-125 Labeled | 10 µCi | $1082 |
| 062-77 | Endotrophin (Mouse) | 20 µg | $382 |
| T-062-77 | Endotrophin (Mouse) - I-125 Labeled | 10 µCi | $1082 |
Social Network Confirmation