
 |
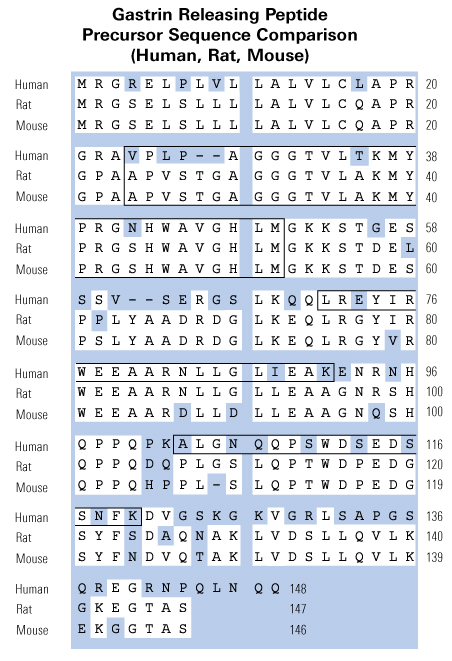 |
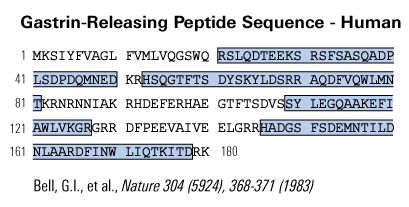
C-terminal fragments from the precursor for gastrin-releasing peptide (GRP) have been detected in several human tumour types. We have previously demonstrated that recombinant human proGRP42-98 is biologically active. To investigate the regions responsible, proGRP42-98 was cleaved with thrombin, and the fragments purified by HPLC. Both proGRP42-79 and proGRP80-98 stimulated proliferation of the human colorectal carcinoma cell line DLD-1, but neither peptide bound to the GRP receptor or bombesin receptor subtype 3. We conclude that two distinct regions of the proGRP C-terminus are biologically active, via a receptor distinct from the known GRP receptors. This discovery opens the way for the development of selective antagonists that may offer new therapies for proGRP-producing tumours.
Patel O, Dumesny C, Shulkes A, Baldwin GS. Recombinant C-terminal fragments of the gastrin-releasing peptide precursor are bioactive. Cancer Lett. 2007;254(1):87-93.
AIMS: To establish whether gastrin releasing peptide (GRP) and the GRP receptor (GRPR) are expressed together in gastrointestinal carcinoid tumours.
METHODS: Twenty six carcinoid tumours from the stomach, small intestine, appendix, and colorectum were investigated by immunohistochemistry for GRP and GRPR.
RESULTS: GRP was detected in nine of 19 tumours and GRPR in 22 of 26. Coexpression of both the ligand and receptor was seen in six of 19 cases. GRPR but not GRP was more strongly expressed in appendix and colonic tumours.
CONCLUSIONS: GRP and GRPR are produced by a large number of gastrointestinal carcinoid tumours. An autocrine/paracrine pathway may exist for GRP stimulated cell proliferation in some of these neoplasms, analogous to that seen in small cell anaplastic carcinoma of the lung.
Scott N, Millward E, Cartwright EJ, Preston SR, Coletta PL. Gastrin releasing peptide and gastrin releasing peptide receptor expression in gastrointestinal carcinoid tumours. Journal of Clinical Pathology. 2004;57(2):189-192.
INTRODUCTION: Symptoms of dyspepsia are common but most patients do not have major upper gastrointestinal pathology. Endoscopy is recommended for dyspeptic patients over the age of 45, or those with certain "alarm" symptoms. We have evaluated the effectiveness of age and "alarm" symptoms for predicting major endoscopic findings in six practising endoscopy centres.METHODS: Clinical variables of consecutive patients with dyspepsia symptoms undergoing upper endoscopy examinations were recorded using a common endoscopy database. Patients who had no previous upper endoscopy or barium radiography were included. Stepwise multivariate logistic regression was used to identify predictors of endoscopic findings. The accuracy of these for predicting endoscopic findings was evaluated with receiver operating characteristic analysis. The sensitivity and specificity of age thresholds from 30 to 70 years were evaluated.RESULTS: Major pathology (tumour, ulcer, or stricture) was found at endoscopy in 787/3815 (21%) patients with dyspepsia. Age, male sex, bleeding, and anaemia were found to be significant but weak independent predictors of endoscopic findings. A multivariate prediction rule based on these factors had poor predictive accuracy (c statistic=0.62). Using a simplified prediction rule of age > or =45 years or the presence of any "alarm" symptom, sensitivity was 87% and specificity was 26%. Increasing or decreasing the age cut off did not significantly improve the predictive accuracy.CONCLUSIONS: Age and the presence of "alarm" symptoms are not effective predictors of endoscopic findings among patients with dyspepsia. Better clinical prediction strategies are needed to identify patients with significant upper gastrointestinal pathology.
Wallace MB, Durkalski VL, Vaughan J, et al. Age and alarm symptoms do not predict endoscopic findings among patients with dyspepsia: a multicentre database study. Gut. 2001;49(1):29-34.

| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| EK-027-07 | Gastrin Releasing Peptide (GRP) (Human) - EIA Kit | 96 wells | $570 |
| 027-40 | Gastrin Releasing Peptide (GRP) (Mouse) | 100 µg | $152 |
| EK-027-04 | Gastrin I (Human) - EIA Kit | 96 wells | $570 |
| FEK-027-07CE | Gastrin Releasing Peptide (GRP) (Human) - Fluorescent EIA Kit, CE Mark Certified | 96 wells | $649 |
| 027-16 | Gastrin Releasing Peptide (GRP) (14-27) (Human, Porcine) | 500 µg | $102 |
| 027-18 | Carboxyl Terminal Flanking Peptide (CTFP) of Progastrin (Rat) | 500 µg | $108 |
| 027-20 | Gastrin (4-17) / Minigastrin (Human) | 200 µg | $108 |
| 027-01 | Gastrin (Chicken) | 100 µg | $230 |
| 027-04 | Gastrin I (Human) | 200 µg | $113 |
| H-027-04 | Gastrin I (Human) - Antibody | 50 µl | $225 |
Social Network Confirmation