The testes secrete four hormones (anti-Müllerian hormone, insulin-like peptide 3, Inhibin B and testosterone) from two endocrine cell types. It is unknown whether anti-Müllerian hormone and insulin-like peptide 3 levels have a diurnal variation, and if so, whether they covary during the day with testosterone and InhB. Sera were obtained from 13 men at 00:00, 06:00, 09:00, 12:00, 14:00, 17:00 and 19:00 hours and the levels of their testicular hormones measured by ELISA. A second cohort of 20 men was similarly examined with blood drawn at 19:00 and the following 06:00. Anti-Müllerian hormone levels exhibited a subtle diurnal pattern with a 19:00 peak that was 4.9% higher on average than the 06:00 nadir (p = 0.004). The decrease in anti-Müllerian hormone coincided with a rise in testosterone and InhB, but there was no association between the person-to-person variation in the diurnal patterns of anti-Müllerian hormone and testosterone or Inhibin B. Insulin-like peptide 3 had no diurnal pattern, with only minor sporadic variation between time points being observed in some men. In conclusion, the diurnal and sporadic variation of each testicular hormone is distinct, indicating that the major regulation is at the level of the hormone rather than at the endocrine cell type. Consequently, the balance of the hormones being released by the testes has complex variation during the day. The physiological significance of this will vary depending on which combinations of testicular hormones that the target cells respond to.
This publication used an INSL3 Fluorescent EIA Kit (catalog#FEK-035-27) from Phoenix Pharmaceuticals.
Chong YH, Pankhurst MW, Mclennan IS. The Daily Profiles of Circulating AMH and INSL3 in Men are Distinct from the Other Testicular Hormones, Inhibin B and Testosterone. PLoS ONE. 2015;10(7):e0133637.
Prenatal sex hormones can induce abnormalities in the reproductive system and adversely impact on genital development. We investigated whether sex hormones in cord blood influenced the ratio of the second to fourth digit lengths (2D/4D) in school-aged children. Of the 514 children who participated in a prospective cohort study on birth in Sapporo between 2002 and 2005, the following sex hormone levels were measured in 294 stored cord blood samples (135 boys and 159 girls); testosterone (T), estradiol (E), progesterone, LH, FSH, inhibin B, and insulin-like factor 3 (INSL3). A total of 350 children, who were of school age and could be contacted for this survey, were then requested via mail to send black-and-white photocopies of the palms of both the left and right hands. 2D/4D was calculated in 190 children (88 boys and 102 girls) using photocopies and derived from participants with the characteristics of older mothers, a higher annual household income, higher educational level, and fewer smokers among family members. 2D/4D was significantly lower in males than in females (p<0.01). In the 294 stored cord blood samples, T, T/E, LH, FSH, Inhibin B, and INSL3 levels were significantly higher in samples collected from males than those from females. A multivariate regression model revealed that 2D/4D negatively correlated with INSL3 in males and was significantly higher in males with <0.32 ng/mL of INSL3 (p<0.01). No correlations were observed between other hormones and 2D/4D. In conclusion, 2D/4D in school-aged children, which was significantly lower in males than in females, was affected by prenatal Leydig cell function.
This publication used an INSL3 EIA Kit (catalog#EK-035-27) from Phoenix Pharmaceuticals.Mitsui T, Araki A, Imai A, et al. Effects of prenatal Leydig cell function on the ratio of the second to fourth digit lengths in school-aged children. PLoS ONE. 2015;10(3):e0120636.
Few studies have been undertaken to assess the possible effects of bisphenol A (BPA) on the reproductive hormone balance in animals or humans with often contradictory results. We investigated possible direct endocrine disruption by BPA of the fetal testes of 2 rat strains (14.5-17.5 days post-coitum) and humans (8-12 gestational weeks) and under different culture conditions. BPA concentrations of 10(-8)M and 10(-5)M for 72 h reduced testosterone production by the Sprague-Dawley fetal rat testes, while only 10-5M suppressed it in the Wistar strain. The suppressive effects at 10-5M were seen as early as 24h and 48 h in both strains. BPA at 10(-7)-10(-5)M for 72 h suppressed the levels of fetal rat Leydig cell insulin-like factor 3 (INSL3). BPA exposure at 10(-8)M, 10(-7)M, and 10(-5)M for 72 h inhibited testosterone production in fetal human testes. For the lowest doses, the effects observed occurred only when no gonadotrophin was added to the culture media and were associated with a poorly preserved testicular morphology. We concluded that (i) BPA can display anti-androgenic effects both in rat and human fetal testes; (ii) it is essential to ascertain that the divergent effects of endocrine disruptors between species in vitro do not result from the culture conditions used, and/or the rodent strain selected; (iii) the optimization of each in vitro assay for a given species should be a major objective rather than the search of an hypothetical trans-species consensual model-system, as the organization of the testis is intrinsically different between mammalian species; (iv) due to the uncertainty existing on the internal exposure of the human fetal testis to BPA, and the insufficient number of epidemiological studies on the endocrine disruptive effects of BPA, caution should be taken in the extrapolation of our present results to the human reproductive health after fetal exposure to BPA.
Ben maamar M, Lesné L, Desdoits-lethimonier C, et al. An investigation of the endocrine-disruptive effects of bisphenol a in human and rat fetal testes. PLoS ONE. 2015;10(2):e0117226.
Context: Insulin-like factor 3 (INSL3) is a testicular hormone secreted during fetal life, the neonatal period, and after puberty. Objective: To measure INSL3 levels in a large series of men with congenital hypogonadotropic hypogonadism (CHH)/ Kallmann syndrome (KS), in order to assess its diagnostic value and to investigate its regulation.
Patients: We studied 281 CHH/KS patients (91 untreated, 96 receiving T, and 94 receiving combined gonadotropin therapy [human chorionic gonadotropin, hCG, and FSH]) and 72 age-matched healthy men.
Methods: Serum INSL3 was immunoassayed with a validated RIA (Phoenix’s cat.#RK-035-27). Results: Mean (±SD) INSL3 levels (pg/mL) were 659 ± 279 in controls and lower (60 ± 43; P < .001) in untreated CHH/KS patients, with no overlap between the two groups, when the threshold of 250 pg/mL was used. Basal INSL3 levels were lower in both untreated CHH/KS men with cryptorchidism than in those with intrascrotal testes and in patients with testicular volumes below 4 mL. Significant positive correlations between INSL3 and both serum total T and LH levels were observed in untreated CHH/KS. Mean INSL3 levels remained low in T-treated CHH/KS patients and were significantly higher in men receiving combined hCG-FSH therapy (P < .001), but the increase was lower cryptorchid patients. FSH-hCG combination therapy or hCG monotherapy, contrary to T and FSH monotherapies, significantly increased INSL3 levels in CHH/KS.
Conclusions: INSL3 is as sensitive a marker as T for the evaluation of altered Leydig cell function in CHH/KS patients. INSL3 levels correlate with LH levels in CHH/KS men showing, together with the rise in INSL3 levels during hCG therapy, that INSL3 secretion seems not constitutively secreted during adulthood but is dependence on pituitary LH.
This publication used an INSL3 RIA Kit (catalog#RK-035-27) from Phoenix Pharmaceuticals.
Trabado S, Maione L, Bry-gauillard H, et al. Insulin-like peptide 3 (INSL3) in men with congenital hypogonadotropic hypogonadism/Kallmann syndrome and effects of different modalities of hormonal treatment: a single-center study of 281 patients. J Clin Endocrinol Metab. 2014;99(2):E268-75.
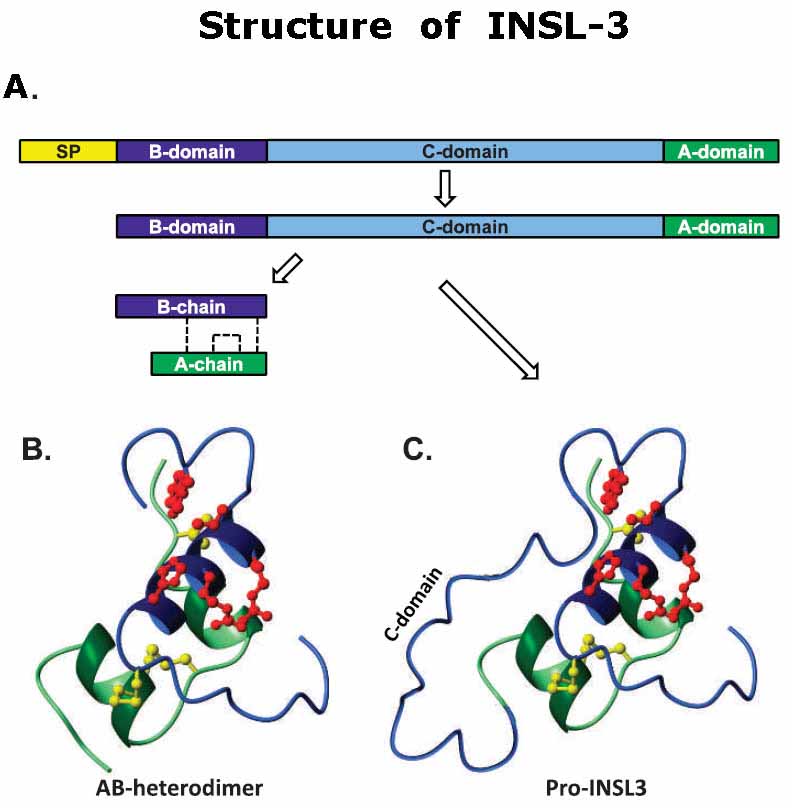
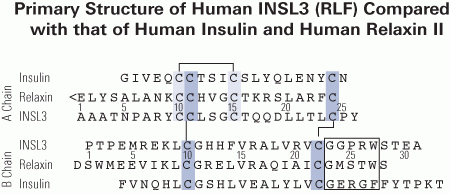
Relaxin-like factor (RLF), also called insulin-like peptide 3 (INSL3), is a member of the insulin/relaxin gene family and is produced by testicular Leydig cells. While the understanding of its effects is growing, very little is known about the structural and functional properties of native INSL3. Here, we demonstrate that native INSL3 isolated from goat testes is a single-chain structure with full biological activity, and is constitutively expressed and secreted by Leydig cells. Using a series of chromatography steps, native INSL3 was highly purified as a single 12-kDa peak as revealed by SDS-PAGE. MS/MS analysis provided 81% sequence coverage and revealed a distinct single-chain structure consisting of the B-, C-, and A-domains deduced previously from the INSL3 cDNA sequence. Moreover, the N-terminal peptide was six amino acid residues longer than predicted. Native INSL3 exhibited full bioactivity in HEK-293 cells expressing the receptor for INSL3. Immunoelectron microscopy and Western blot analysis revealed that INSL3 was secreted by Leydig cells through the constitutive pathway into blood and body fluids. We conclude, therefore, thatgoat INSL3 is constitutively secreted from Leydig cells as a B-C-A single-chain structure with full biological activity.
Siqin, Minagawa I, Okuno M, et al. The active form of goat insulin-like peptide 3 (INSL3) is a single-chain structure comprising three domains B-C-A, constitutively expressed and secreted by testicular Leydig cells. Biol Chem. 2013;394(9):1181-94.
STUDY QUESTION: How does insulin-like factor 3 (INSL3) concentration in blood vary across the menstrual cycle in women? SUMMARY ANSWER: INSL3 is secreted by the theca interna cells of growing antral follicles and is phasic in its expression.
WHAT IS KNOWN ALREADY: The relaxin-like hormone INSL3 is known to be expressed in follicles of several mammal species, and was recently shown in cows to be specifically secreted into the bloodstream by growing antral follicles, corresponding to follicular waves. In males INSL3 is known to be acutely independent of the hormones of the hypothalamic-pituitary-gonadal axis, suggesting that in women INSL3 might be a novel biomarkerfor antral follicle recruitment and development.
STUDY DESIGN, SIZE, DURATION: Two cohorts of women were studied. First, 18 healthy women of reproductive age were followed longitudinally for one and a half cycles, with blood sampling and hormone measurement every 2-3 days. A second cohort comprised a cross-sectional study of 909 women attending an infertility clinic, with a single blood sample taken at entry, together with other clinical and hormonal parameters. PARTICIPANTS/MATERIALS, SETTING, METHODS: Blood samples from both retrospective cohorts were analyzed for INSL3 using a highly sensitive time-resolved fluorescent immunoassay, and data were analyzed in comparison with other clinical and hormonal parameters. MAIN RESULT AND THE ROLE OF CHANCE: For young healthy women of reproductive age, we showed a phasic expression of INSL3 corresponding to antral follicle growth in both the follicular and luteal phases of the cycle, which was significantly (P < 0.05) elevated compared with that during menses. For women attending an infertility clinic, those with diagnosed polycystic ovarian syndrome indicated significantly (P < 0.0005) greater circulating INSL3 levels and those with low ovarian reserve showed significantly (P < 0.002) decreased INSL3 values.
LIMITATIONS, REASONS FOR CAUTION: These were retrospective studies and the results were obtained from natural cycles only, with their inherent variability.
WIDER IMPLICATIONS OF THE FINDINGS: We show for the first time that INSL3 in women does vary across the menstrual cycle, and appears to reflect the number of growing antral follicles recruited within both follicular and luteal phases.
STUDY FUNDING/COMPETING INTEREST(S): The present retrospective study was largely supported by departmental funds. There were no competing interests.
Anand-ivell R, Tremellen K, Dai Y, et al. Circulating insulin-like factor 3 (INSL3) in healthy and infertile women. Hum Reprod. 2013;28(11):3093-102.
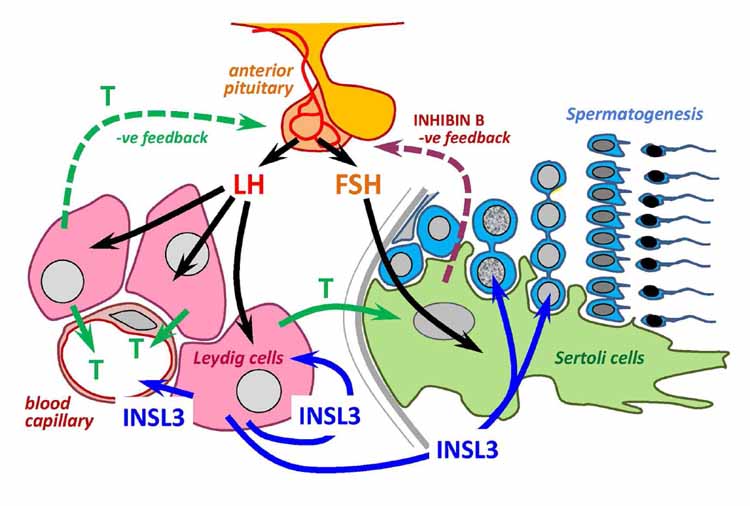
Insulin-like factor 3 (INSL3) is a small peptide hormone made and secreted uniquely by mature Leydig cells in the testes of all mammals. Importantly, this expression and secretion appears to be constitutive and therefore reflects the differentiation status and number of the Leydig cells present, differing thereby from testosterone, which is acutely and homeostatically regulated by the hormones of the hypothalamic-pituitary-gonadal axis. As a consequence, the measurement of INSL3 either as mRNA in the testis or as secreted peptide circulating in the blood provides an excellent assessment of Leydig cell differentiation, for example, during fetal development, puberty, or aging or following exposure to endocrine-disrupting agents. Whereas INSL3 is proving increasingly useful as a biomarker for testis status, less is known about its functions, particularly in the adult male. Current evidence points to autocrine, paracrine, and endocrine roles, acting through the G-protein-coupled receptor called RXFP2, although more research is required to characterize these functions in detail.
Ivell R, Wade JD, Anand-ivell R. INSL3 as a biomarker of Leydig cell functionality. Biol Reprod. 2013;88(6):147.
BACKGROUND: Cryptorchidism, incomplete pubertal development, and low testosterone are manifestations of hypogonadism in Prader-Willi syndrome (PWS). Insulin-like peptide-3 (INSL3) facilitates testicular descent in the fetus and reflects Leydig cell number in adults. INSL3 levels in PWS have not been previously reported.
OBJECTIVES: The objectives of the study were to characterize the age-related changes in INSL3 in PWS males and correlate INSL3 with unilateral vs. bilateral cryptorchidism, body mass index, gonadotropins, testosterone, anti-mullerian hormone (AMH), and inhibin B.
STUDY DESIGN AND PARTICIPANTS: We measured INSL3, LH, FSH, testosterone, AMH, and inhibin B in 40 PWS males (23 deletion, 17 uniparental disomy) aged 2 months to 36 yr. Control samples for INSL3 were obtained from 365 normal males, aged 1 d to 36 yr.
RESULTS: INSL3 levels (mean and range) for PWS age groups younger than 6 months, 0.5-10.0 yr, 10.1-19.0 yr, and older than 19.0 yr were 217 (68-380), 42 (16-112), 390 (16-1028), and 642 (290-964) pg/ml, respectively, and did not differ significantly from values for normal males. In seven of 14 boys aged 10.1-19 yr, INSL3, testosterone, and LH were low (37.4 ± 19.4 pg/ml, 1.44 ± 0.46 nmol/liter, 0.3 ± 0.6 IU/liter). The other seven with higher INSL3, testosterone, and LH (693.1 ± 305.8 pg/ml, 5.91 ± 2.77 nmol/liter, 2.7 ±1.9 IU/liter) had more advanced pubertal development. INSL3was normal in seven of nine males aged older than 19 yr, despite low testosterone in six. After controlling for age, INSL3 correlated with LH (P = 0.005) and testosterone (P < 0.001) but not with FSH, AMH, or inhibin B.
CONCLUSIONS: Most PWS males have normal INSL3 levels. By contrast, testosterone levels after infancy are low. These findings suggest a specific defect in Leydig cell function.
This publication used an INSL3 peptide and EIA Kit (catalog#035-27 and EK-035-27, respectively) from Phoenix Pharmaceuticals.
Hirsch HJ, Eldar-geva T, Gross-tsur V, Benarroch F, Roger M, Lahlou N. Normal insulin-like peptide-3 levels despite low testosterone in adult males with Prader-Willi syndrome: variations in Leydig cell function from infancy through adulthood. J Clin Endocrinol Metab. 2013;98(1):E135-43.
PURPOSE OF REVIEW: Biomarkers of prepubertal testicular function have become widely available only in recent years. The aim of this review is to update the knowledge on key biomarkers used to assess hypogonadism in boys.RECENT FINDINGS: Sertoli cells are the most representative cells of the prepubertal testis. Anti-Müllerian hormone and inhibin B are essential biomarkers of Sertoli cell function. Also, INSL3 arises as an additional marker of Leydig cell dysfunction.SUMMARY: The widespread use of these biomarkers has enhanced our knowledge on the pathophysiology and diagnosis of prepubertal male hypogonadism. Beyond their well known germ-cell toxicity, oncologic treatments may also affect Sertoli cell function. Pathophysiology is not the same in all aneuploidies leading to infertility: while hypogonadism is not evident until mid-puberty in Klinefelter syndrome, it is established in early infancy in Down syndrome. In Noonan syndrome, the occurrence of primary hypogonadism depends on the existence of cryptorchidism, and Prader-Willi syndrome may present with either primary or combined forms of hypogonadism. Prepubertal testicular markers have also provided insights into the effects of environmental disruptors on gonadal function from early life, and helped dissipate concerns about testicular function in boys born preterm or small for gestational age or conceived by assisted reproductive technique procedures.
Valeri C, Schteingart HF, Rey RA. The prepubertal testis: biomarkers and functions. Curr Opin Endocrinol Diabetes Obes. 2013;20(3):224-33.
Neohormone systems are defined as evolutionarily new endocrine or paracrine adaptations that supplement basic physiologic functions and define mammalian success. The relaxin family of peptide hormones are typical neohormones. Because they define the specific mammalian aspects of reproductive physiology, such as viviparity with implantation and placentation, lactation, or in the male the necessary adaptations to sperm needed for successful internal fertilization, they offer excellent biomarkers for characterizing reproductive health and disease. For example, ovarian H2-relaxin aids implantation and the establishment of the placenta, and circulating levels are significantly altered in early miscarriage. In the fetus, testicular INSL3 is responsible for the first phase of testicular descent and may be disrupted in cryptorchidism. In the adult, INSL3 is believed to be involved as an antiapoptotic factor in germ cell survival (male) and follicle selection (female) and acts as an excellent measure of Leydig cell functional capacity, particularly in the aging male. INSL5 and INSL6 appear also to be involved in the maintenance of adequate spermatogenesis. With the development of robust immunoassays for various relaxin family members, we are progressively gathering baseline information about normalbiomarker levels as well as their perturbations in a wide range of reproductive pathologies.
Anand-ivell R, Dai Y, Ivell R. Neohormones as biomarkers of reproductive health. Fertil Steril. 2013;99(4):1153-60.
The relaxin peptides are a family of hormones that share a structural fold characterized by two chains, A and B, that are cross-braced by three disulfide bonds. Relaxins signal through two different classes of G-protein-coupled receptors (GPCRs), leucine-rich repeat-containing GPCRs LGR7 and LGR8 together with GPCR135 and GPCR142, now referred to as the relaxin family peptide (RXFP) receptors 1-4, respectively. Although key binding residues have been identified in the B-chain of the relaxin peptides, the role of the A-chain in their activity is currently unknown. A recent study showed that INSL3 can be truncated at the N terminus of its A-chain by up to 9 residues without affecting the binding affinity to its receptor RXFP2 while becoming a high affinity antagonist. This suggests that the N terminus of the INSL3 A-chain contains residues essential for RXFP2 activation. In this study, we have synthesized A-chain truncated human relaxin-2 and -3 (H2 and H3) relaxin peptides, characterized their structure by both CD and NMR spectroscopy, and tested their binding and cAMP activities on RXFP1, RXFP2, and RXFP3. In stark contrast to INSL3, A-chain-truncated H2 relaxin peptides lost RXFP1 and RXFP2 binding affinity and concurrently cAMP-stimulatory activity. H3 relaxin A-chain-truncated peptides displayed similar properties on RXFP1, highlighting a similar binding mechanism for H2 and H3 relaxin. In contrast, A-chain-truncated H3 relaxin peptides showed identical activity on RXFP3, highlighting that the B-chain is the sole determinant of the H3 relaxin-RXFP3 interaction. Our results provide new insights into the action of relaxins and demonstrate that the role of the A-chain for relaxin activity is both peptide- and receptor-dependent.
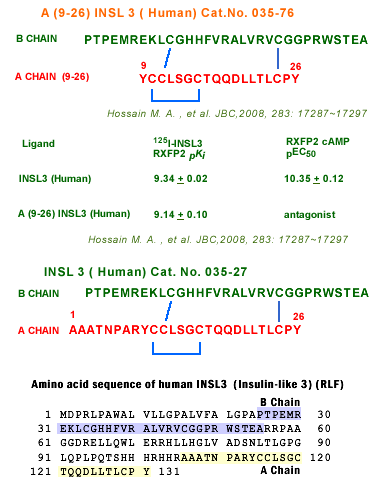
Hossain MA, Rosengren KJ, Haugaard-jönsson LM, et al. The A-chain of human relaxin family peptides has distinct roles in the binding and activation of the different relaxin family peptide receptors. J Biol Chem. 2008;283(25):17287-97.
Testicular descent is a unique physiological adaptation found in therian mammals allowing optimal spermatogenesis below core body temperature. Recent studies show that INSL3, produced by Leydig cells, and its receptor LGR8 (RXFP2) are essential for mediating the transabdominal phase of testicular descent during early development. However, the origin and genetic basis for this physiological adaptation is not clear. Using syntenic mapping and the functional characterization of contemporary and resurrected relaxin family hormones, we show that derivation of INSL3-mediated testicular descent involved the duplication of an ancestral RLN3-like gene that encodes an indiscriminate ligand for LGR7 (RXFP1) and LGR8. This event was followed by acquisition of the LGR7-selective characteristics by a daughter gene (RLN3) prior to the evolution of the common ancestor of monotremes, marsupials, and placentals. A subsequent mutation of the other daughter gene (INSL3) occurred before the emergence of therian mammals, which then led to the derivation of the reciprocal LGR8-specific characteristics of INSL3. The stepwise evolution of these independent signaling pathways through gene duplication and subsequent divergence is consistent with Darwinian theory of selection and adaptation, and the temporal proximity suggests an association between these genetic events and the concurrent evolution of testicular descent in ancestral therian mammals.
Park JI, Semyonov J, Chang CL, Yi W, Warren W, Hsu SY. Origin of INSL3-mediated testicular descent in therian mammals. Genome Res. 2008;18(6):974-85.
The administration of testosterone plus a progestogen functions as a male contraceptive by inhibiting the release of pituitary gonadotropins. After 3 to 4 months of treatment, most men are azoospermic or severely oligospermic (1 million sperm/mL). However, 10% to 20% of men have persistent sperm production despite profound gonadotropin suppression. Since insulin-like factor 3 (INSL3) has been shown to prevent germ cell apoptosis in mice, we hypothesized that INSL3 might be higher in men with persistent spermatogenesis during treatment with male hormonal contraceptives. In a retrospective analysis, we measured serum INSL3 in 107 men from 3 recent male hormonal contraceptive studies and determined the relationship between suppression of spermatogenesis and serum INSL3. At the end of treatment 63 men (59%) were azoospermic and 44 men (41%) had detectable sperm in their ejaculates. Baseline INSL3 did not predict azoospermia; however, end of treatment serum INSL3 was significantly higher in nonazoospermic men compared with those with azoospermia (median [interquartile range]: 95 [73–127] pg/mL vs 80 [67–101] pg/mL; P = .03). Furthermore, serum INSL3 was positively correlated with sperm concentration (r = .25; P = .009) at the end of treatment and was significantly associated with nonazoospermia by multivariate logistic regression (P = .03). After 6 months of treatment with a hormonal male contraceptive regimen, higher serum INSL3 concentrations were associated with persistent sperm production. INSL3 may play a role in preventing complete suppression of spermatogenesis in some men on hormonal contraceptive regimens. This finding suggests that INSL3 may be a potential target for male contraceptive development.
Amory JK, Page ST, Anawalt BD, Coviello AD, Matsumoto AM, Bremner WJ. Elevated end-of-treatment serum INSL3 is associated with failure to completely suppress spermatogenesis in men receiving male hormonal contraception. J Androl. 2007;28(4):548-54.
Serum samples for immunoreactive INSL3 concentration analysis were assayed in duplicate by using a commercial RIA (Phoenix Pharmaceutical, Belmont, CA). The assay is based on a polyclonal rabbit antiserum raised against full-length human INSL3 and on 125I-labeled INSL3 as the tracer. The assay performance was checked before analysis by verifying specificity, sensitivity, precision, and accuracy. To detect the specificity, a cross-reactivity test was conducted via a binding assay with decreasing concentrations of human insulin, INSL4, INSL5, INSL6, and INSL7. The sensitivity was determined as the lower detection limit (28). The precision was checked by using replicates of a serum pool control to measure intraassay and interassay variability. Finally, to detect the accuracy of the method, linearity of dilution was determined by serially diluting serum pool control, and recovery was evaluated by measuring pooled serum samples spiked with increasing standard human INSL3 concentrations before analysis in the RIA. Cross-reactivity with human insulin, INSL4, INSL5, INSL6, and INSL7, used to evaluate the specificity of the INSL3 assay, was 0%. The sensitivity determined as the lower detection limit was 1.8 pg/ml. The intraassay and interassay coefficients of variation were 3.1 and 6.8%, respectively. Suitability of the assay to measure INSL3 accurately was demonstrated by results of linearity and recovery (slope of 1; mean of recovery of 104%), which demonstrated the absence of bias.
Gambineri A, Patton L, De iasio R, Palladoro F, Pagotto U, Pasquali R. Insulin-like factor 3: a new circulating hormone related to luteinizing hormone-dependent ovarian hyperandrogenism in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(6):2066-73.
Context Insulin-like factor 3 (INSL3) is produced by the Leydig cells and in adults its secretion is dependent on the state of differentiation of these cells, which, in turn, is dependent on LH. However, the secretion and regulation of INSL3 during puberty is unknown. Objective To evaluate INSL3 concentrations during normal male puberty and its relation to LH, FSH and testosterone. Design Cross-sectional study conducted from January to December 2005. Setting Academic clinics Patients 75 healthy male subjects aged 9.5-17.5 yr, homogeneously distributed into five pubertal groups of 15 according to Tanner stages. Interventions None Main outcome measures Mean testicular volume and LH, FSH, testosterone and INSL3 concentrations in relation to age and pubertal stage. Results We observed an increase of INSL3 and LH levels from Tanner stage 2 to 4, and an increase of FSH from stage 2 to 3. Testosterone levels increased from stage 3 to 4. No differences were seen for all measured hormones between stage 4 and 5. The increase in INSL3 seems therefore to anticipate the increase in testosterone. However, INSL3 plasma concentrations at pubertal stages 4 and 5 are about one fourth of adult levels, whereas FSH, LH and testosterone reached adult levels by stage 4. Positive significant correlations were found between INSL3 and LH for all pubertal stages. Conclusions This study provides information on the physiological dynamics of INSL3 showing that the serum concentrations of this hormone increased progressively throughout puberty, under the differentiating action of LH on Leydig cells. INSL3 is therefore confirmed to represent a marker of Leydig cell differentiation and function. However, a prolonged exposition to LH seems to be necessary to reach INSL3 concentrations of adults. A possible use of INSL3 in puberty disorders is promising.
Ferlin A, Garolla A, Rigon F, Rasi caldogno L, Lenzi A, Foresta C. Changes in serum insulin-like factor 3 during normal male puberty. J Clin Endocrinol Metab. 2006;91(9):3426-31.
Insulin-like factor 3 (INSL3) plays a crucial role in testicular descent. Genetic ablation of Insl3 or its G protein-coupled receptor, leucine-rich repeat-containing G-protein-coupled receptor (Lgr8), causes cryptorchidism in mice. Mutation analyses of INSL3 in humans showed an association with cryptorchidism but led to non-conclusive data about a causative role. In this study, we explored the hypothesis that mutations in INSL3 may be associated with the signs of testicular dysgenesis syndrome (TDS). We screened for mutations in INSL3 gene in 967 subjects with a history of maldescended testes and/or infertility and/or testicular cancer and in 450 controls. Furthermore, we carried out in vitro functional analysis of three novel mutations by analysis of INSL3-dependent cAMP increase in cells expressing LGR8. We found six INSL3 mutations in 18 of 967 patients (1.9%) and no mutations in controls. Prevalence of mutations was similar in the different groups of patients (cryptorchidism and/or infertility and/testicular cancer). Three mutations were novel findings (R4H, W69R, and R72K); however, their analysis showed normal cAMP increase after the activation of LGR8 receptor. In conclusion, we found a significant association of INSL3 gene mutations in men presenting one or more signs of TDS syndrome. However, a causative role for some of these mutations is not clearly supported by functional analyses. Although a role for mutations of INSL3 and LGR8 genes in cryptorchidism is reasonable, additional studies are needed to establish an association between the disruption of INSL3 pathway and higher risk of infertility or testicular cancer.
Ferlin A, Bogatcheva NV, Gianesello L, et al. Insulin-like factor 3 gene mutations in testicular dysgenesis syndrome: clinical and functional characterization. Mol Hum Reprod. 2006;12(6):401-6.
CONTEXT: Gonadotropic regulation of the testicular Leydig cell hormone insulin-like factor 3 (INSL3) is incompletely characterized.OBJECTIVE: The objective of this study was to assess the effects of gonadotropin suppression and induced or spontaneous recovery on serum INSL3.DESIGN AND PARTICIPANTS: Serum samples from 15 men enrolled in a short-term study of gonadotropin stimulation, suppression, and recovery and 11 men in a long-term study of gonadotropin suppression and spontaneous recovery were analyzed for INSL3. Intervention: Gonadotropins were suppressed by exogenous testosterone and progestin. Recovery was spontaneous or induced with exogenous gonadotropins.
OUTCOME MEASURE: The outcome measure was serum INSL3 in relation to other reproductive hormones.RESULTS: Serum INSL3 was not acutely sensitive to gonadotropins. In both studies, INSL3 declined markedly with gonadotropin suppression (6-13.5% of baseline; P < 0.05). In the short-term study, human chorionic gonadotropin partially restored suppressed serum INSL3 within 4 d of administration (from 7.5 to 38.3% baseline; P < 0.05); the increase correlated with the corresponding increase in serum pro-alphaC (r = 0.82; P < 0.01). FSH did not stimulate the suppressed INSL3. In the long-term study, serum testosterone recovered significantly better (80% baseline) compared with serum INSL3 (38.9% baseline; P < 0.01) in the presence of fully recovered serum LH.CONCLUSIONS: INSL3 is not sensitive to gonadotropin stimulation in normal men, but declines markedly in response to gonadotropin deprivation. After suppression, INSL3 was responsive to hCG 4 d after administration. After long-term suppression, INSL3 did not recover to the same degree as testosterone, suggesting that INSL3 is more sensitive to Leydig cell impairment than testosterone.
Bay K, Matthiesson KL, Mclachlan RI, Andersson AM. The effects of gonadotropin suppression and selective replacement on insulin-like factor 3 secretion in normal adult men. J Clin Endocrinol Metab. 2006;91(3):1108-11.
Insulin-like 3 (INSL3) is a hormone produced by testicular Leydig cells throughout life. During embryonic life it regulates an essential step of testicular descent, whereas in adults it acts as a male germ cell survival factor. Despite the importance of INSL3 for male sex differentiation and function, very little is known regarding the molecular mechanisms that regulate its expression. So far, the nuclear receptor SF-1 is the only transcription factor known to regulate the mouse Insl3 promoter in Leydig cells. In order to further our understanding of the transcriptional regulation of INSL3 expression, we have isolated the human INSL3 promoter and tested the effects of the nuclear receptors SF-1, LRH-1, and Nur77 on its activity in Leydig cells. In transfections assays, all three nuclear receptors activated the human INSL3 promoter but especially Nur77, which acted through a novel regulatory element. Thus, the human INSL3 promoter constitutes a novel target for the orphan nuclear receptor Nur77.
Tremblay JJ, Robert NM. Role of nuclear receptors in INSL3 gene transcription in Leydig cells. Ann N Y Acad Sci. 2005;1061:183-9.
The human prostate gland is a well known source of H1 and H2 relaxin but information is lacking on the expression and potential role of the INSL3 peptide hormone within the prostate gland. In the present study we have investigated the expression of human INSL3 in patients with benign prostate hyperplasia (BPH), prostate intraepithelial neoplasia (PIN) and prostate carcinoma tissues. Of the prostate epithelial cells, strongest INSL3 expression was detected in the basal epithelial cell compartment. Weaker INSL3 mRNA expression and immunoreactive INSL3 production were observed in secretory epithelial cells and in interstitial smooth muscle cells. Prostate epithelial cells were also a source for transcripts encoding the INSL3 receptor LGR8 suggesting the presence of an autocrine/paracrine INSL3-LGR8 ligand-receptor system within the human prostate. Three human prostate carcinoma cell lines displayed differential gene activity for INSL3 and LGR8. While LNCaP was devoid of INSL3, the androgen-insensitive PC-3 and the stromal prostate cell line hPCP co-expressed INSL3 and LGR8 transcripts. In addition to expressing INSL3 mRNA, the LGR8-negative DU-145 also expressed an INSL3 splice form formerly demonstrated in thyroid carcinoma cells. When incubated with recombinant INSL3, PC-3 cells showed significantly increased intracellular cAMP levels indicating functional LGR8 receptors. INSL3 did not alter the proliferative or metabolic activity of PC-3 carcinoma cells. Instead, PC-3 responded to INSL3 with significantly enhanced tumor cell motility and a transcriptional down-regulation of ErbB receptors and EGF. All-trans-retinoic acid was demonstrated in PC-3 to up-regulate LGR8 gene activity in a dose- and time-dependent manner while having no effect on INSL3 gene activity. In conclusion, we have identified a functional INSL3-LGR8 ligand-receptor system in human prostate carcinoma cells.
Klonisch T, Müller-huesmann H, Riedel M, et al. INSL3 in the benign hyperplastic and neoplastic human prostate gland. Int J Oncol. 2005;27(2):307-15.
Insulin-like factor 3 (INSL3) is a member of the relaxin-insulin family, and it is expressed in pre- and postnatal Leydig cells of the testis. This peptide affects testicular descent during embryonic development, and mutations in INSL3 gene or its receptor LGR8 (leucine-rich repeat-containing G protein-coupled receptor 8)/GREAT (G protein-coupled receptor affecting testicular descent) cause cryptorchidism in humans. The expression of LGR8/GREAT in different tissues and the production of INSL3 also by adult-type Leydig cells suggest additional roles of this hormonal system in adulthood. In this preliminary report we performed the first analysis in humans of INSL3 using a novel RIA kit to measure INSL3 concentrations in serum of normal men and with different testicular pathologies. The results show that INSL3 is circulating in adult men, and it is almost exclusively of testicular origin. Subjects with severe testicular damage, such as men with severe infertility, produce low amount of INSL3, and the concentrations of this hormone seem to reflect the functional status of the Leydig cells. In particular, INSL3 concentrations may be an even more sensitive marker of Leydig cell function than testosterone itself. Analysis of men treated with different combinations of hormones of the hypothalamus-pituitary-testis axis suggests that the production of INSL3 is related to LH in a manner similar to that of the LH-testosterone axis.
Foresta C, Bettella A, Vinanzi C, et al. A novel circulating hormone of testis origin in humans. J Clin Endocrinol Metab. 2004;89(12):5952-8.
Testicular descent is a complex multistep embryonic process requiring the interaction between anatomical and hormonal factors. Failure in any of these steps results in cryptorchidism, the most frequent congenital anomaly of the urogenital tract in human males. Evidence for a genetic cause for cryptorchidism is numerous and supported by animal models. In particular, INSL3 and LGR8/GREAT proteins seem to act as ligand and receptor, respectively, and to have a role in gubernaculum development involved in testicular descent. In a cohort of 87 ex-cryptorchid patients and 80 controls, we looked for mutations in INSL3 and LGR8/GREAT genes by sequencing. Patients were classified on the basis of seminal, hormonal, and testicular cytological analyses. We found three mutations in the INSL3 gene in four patients and one LGR8/GREAT mutation in four patients (8 of 87, 9.2%). The eight patients show different phenotypes, ranging from normozoospermia to complete azoospermia, and from bilateral cryptorchidism to retractile testes. Furthermore, the endocrine function of the testis appears normal in all subjects. The findings of our study demonstrate that INSL3-LGR8/GREAT mutations are frequently associated with human cryptorchidism and are maternally inherited. The only clinical consequence of alterations of the INSL3-LGR8/GREAT system seems to be failure of the testis to normally descend in the scrotum during embryonic development, without affecting the spermatogenic and endocrine components of the testis itself.
Ferlin A, Simonato M, Bartoloni L, et al. The INSL3-LGR8/GREAT ligand-receptor pair in human cryptorchidism. J Clin Endocrinol Metab. 2003;88(9):4273-9.
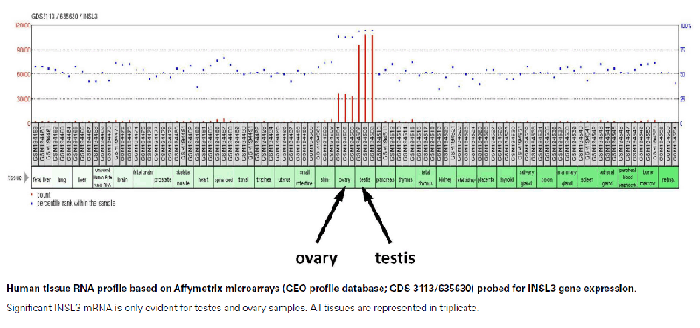
Insulin-like protein (INSL3, INSL4, INSL5, INSL6 & INSL7/H3 Relaxin) belongs to the insulin-like hormone superfamily, which encompasses insulin, relaxin, and insulin-like growth factors I (IGF1) and II (IGF2). Insulin gene superfamily hormones regulate cell growth, metabolism, and tissue-specific functions. Members of this family are characterized by a signal peptide, a B chain, a connecting C chain, and an A chain. A preliminary in-house study performed at Phoenix Pharmaceuticals, Inc. found gender specific applications for INSL3. Results from analysis using INSL3 RIA Kit (RK-035-27) showed the levels of circulating INSL3 in male samples were approximately ten times greater than that found in female samples.
April 18, 2003 Phoenix Pharmaceuticals, Inc. Belmont, CA
We have recently identified the insulin-like peptide relaxin-3 (aka INSL7) as the endogenous ligand for an orphan G-protein-coupled receptor, GPCR135 (aka somatostatin- and angiotensin-like peptide receptor). Analysis of possible receptors related to GPCR135 revealed a single orphan receptor, GPCR142. Thus, we tested whether GPCR142 could also respond to relaxin-3 or related insulin-like molecules. Surprisingly, GPCR142 was activated by nanomolar concentrations of relaxin-3 but was completely unresponsive to all other known insulin-like peptides. We evaluated by reverse transcriptase-PCR the expression of GPCR142 mRNA in a variety of human tissues and found expression in brain, kidney, testis, thymus, placenta, prostate, salivary gland, thyroid, and colon. In an analysis of other species, we were able to find a full-length mouse homolog of GPCR142, but were unable to detect any complete GPCR142 transcripts in rat. With respect to intracellular signaling, GPCR142 is similar to GPCR135 in that it potently inhibits adenylate cyclase and stimulates 35S-GTPgammaS incorporation in response to relaxin-3. However, whereas GPCR135 signaling could be converted to calcium mobilization using a Gqi5 or Galpha16 G-proteins, GPCR142 was only capable of functioning in the presence of Galpha16. In the accompanying article (Liu, C., Eriste, E., Sutton, S., Chen, J., Roland, B., Kuei, C., Farmer, N., Jornvall, H., Sillard, R., and Lovenberg, T. W. (2003) J. Biol. Chem. 278, 50754-50764), we present the case that relaxin-3, which has previously been shown to bind to the relaxin receptor LGR7, is most likely the endogenous ligand for GPCR135. In this report, we show an additional receptor, GPCR142, which is also selectively activated by relaxin-3. However, the anatomical localization of GPCR142 suggests that GPCR142 may have different physiological functions.
Liu C, Chen J, Sutton S, et al. Identification of relaxin-3/INSL7 as a ligand for GPCR142. J Biol Chem. 2003;278(50):50765-70.
The omnipresent 6kDa polypeptide relaxin (RLX) is emerging as a multi-functional endocrine and paracrine factor, with a broad range of target tissues that includes the cardiovascular system. Humans and other higher primates have three RLX genes, designated H1, H2 and H3, of which H2 RLX is the major stored and circulating form. Rodents have only two RLX genes: relaxin-1 (equivalent to H2 RLX) and relaxin-3 (equivalent to H3 RLX). The recent cloning of the human RLX receptor (LGR7), a member of the leucine-rich repeat family of G-protein-coupled orphan receptors, and detection of LGR7 gene transcripts in the heart confirm this organ as a target for RLX (H2). However, evidence for production of the ligand within the cardiovascular system is limited, and few studies have clearly identified the physiological effects of RLX on cardiac function. To add to the controversy, serum concentrations and expression of RLX in the heart are elevated in chronic heart failure patients and animal models of cardiomyopathy, implying that RLX may only be a marker for pathological cardiovascular conditions, rather than normal physiology.
Samuel CS, Parry LJ, Summers RJ. Physiological or pathological--a role for relaxin in the cardiovascular system?. Curr Opin Pharmacol. 2003;3(2):152-8.
The insulin-like hormone INSL-3, also named relaxin-like factor (RLF) or Leydig-derived insulin-like peptide (LEY-IL), is expressed in various reproductive tissues and is regarded a marker of differentiation in human testicular Leydig cells. Recently, we have identified differential expression of human INSL-3 in neoplastic Leydig cells and mammary epithelial cells suggesting an involvement of INSL-3 in tumor biology. Here we have investigated the expression of INSL-3 in human thyroid carcinoma cell lines and in the human thyroid gland which has been shown to express transcripts for the G protein coupled INSL-3 receptor LGR8. When we determined the expression of INSL-3 in eight human thyroid carcinoma cell lines, a novel INSL-3 splice variant containing a 95 bp out-of-frame insertion at the beginning of exon II of the INSL-3 gene was discovered. Treatment of the human anaplastic thyroid carcinoma cell line 8505C with diethylstilbestrol (DES) caused a significant dose-dependent transcriptional down-regulation of INSL-3 and a marked up-regulation of LGR8. Employing in situ hybridization to detect INSL-3 transcripts and specific rabbit antisera against the INSL-3 proteins, both INSL-3 isoforms were detected in patients with Graves' disease (n=10), follicular carcinomas (FTC; n=12), papillary carcinomas (PTC; n=9) and undifferentiated anaplastic carcinomas (UTC; n=15). By contrast, thyrocytes of all 15 benign goiter tissues studied were devoid of both INSL-3 isoforms, mRNA and protein. Our data indicate that INSL-3 hormone is up-regulated in hyperplastic and neoplastic human thyrocytes suggesting that the INSL-3 isoforms may serve as additional markers for hyperplastic and neoplastic human thyrocytes. In the anaplastic thyroid carcinoma cell line 8505C, the regulation of both INSL-3 and LGR8 by estrogen may be the first indication of a novel hormonally responsive, auto-/paracrine INSL-3 LGR8 ligand receptor system active in human thyroid carcinoma cells.
Hombach-klonisch S, Hoang-vu C, Kehlen A, et al. INSL-3 is expressed in human hyperplastic and neoplastic thyrocytes. Int J Oncol. 2003;22(5):993-1001.
Leucine-rich repeat-containing, G protein-coupled receptors (LGRs) represent a unique subgroup of G protein-coupled receptors with a large ectodomain. Recent studies demonstrated that relaxin activates two orphan LGRs, LGR7 and LGR8, whereas INSL3/Leydig insulin-like peptide specifically activates LGR8. Human relaxin 3 (H3 relaxin) was recently discovered as a novel ligand for relaxin receptors. Here, we demonstrated that H3 relaxin activates LGR7 but not LGR8. Taking advantage of the overlapping specificity of these three ligands for the two related LGRs, chimeric receptors were generated to elucidate the mechanism of ligand activation of LGR7. Chimeric receptor LGR7/8 with the ectodomain from LGR7 but the transmembrane region from LGR8 maintains responsiveness to relaxin but was less responsive to H3 relaxin based on ligand stimulation of cAMP production. The decreased ligand signaling was accompanied by decreases in the ability of H3 relaxin to compete for 33P-relaxin binding to the chimeric receptor. However, replacement of the exoloop 2, but not exoloop 1 or 3, of LGR7 to the chimeric LGR7/8, restored ligand binding and receptor-mediated cAMP production. These results suggested that activation of LGR7 by H3 relaxin involves specific binding of the ligand to both the ectodomain and the exoloop 2, thus providing a model with which to understand the molecular basis of ligand signaling for this unique subgroup of G protein-coupled receptors.
Sudo S, Kumagai J, Nishi S, et al. H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem. 2003;278(10):7855-62.
Relaxin is a peptide hormone with known actions associated with female reproductive physiology, but it has also been identified in the brain. Only one relaxin gene had been characterized in rodents until recently when a novel human relaxin gene, human gene-3 (H3) and its mouse equivalent (M3) were identified. The current study reports the identification of a rat homologue, rat gene-3 (R3) relaxin that is highly expressed in a discrete region of the adult brain. The full R3 relaxin cDNA was generated using RT-PCR and 3' and 5' RACE protocols. The derived amino acid sequence of R3 relaxin retains all the characteristic features of a relaxin peptide and has a high degree of homology with H3 and M3 relaxin. The distribution of R3 relaxin mRNA in adult rat brain was determined and highly abundant expression was only detected in neurons of the ventromedial dorsal tegmental nucleus (vmDTg) in the pons, whereas all other brain areas were unlabelled or contained much lower mRNA levels. Relaxin binding sites and relaxin immunoreactivity were also detected in the vmDTg. These together with earlier findings provide strong evidence for a role(s) for multiple relaxin peptides as neurotransmitters and/or modulators in the rat CNS.
Burazin TC, Bathgate RA, Macris M, Layfield S, Gundlach AL, Tregear GW. Restricted, but abundant, expression of the novel rat gene-3 (R3) relaxin in the dorsal tegmental region of brain. J Neurochem. 2002;82(6):1553-7.
Relaxin is an insulin-like peptide consisting of two separate chains (A and B) joined by two inter- and one intrachain disulfide bonds. Binding to its receptor requires an Arg-X-X-X-Arg-X-X-Ile motif in the B-chain. A related member of the insulin superfamily, INSL3, has a tertiary structure that is predicted to be similar to relaxin. It also possesses an Arg-X-X-X-Arg motif within its B-chain, although this is displaced by four amino acids towards the C-terminus from the corresponding position within relaxin. We have previously shown that synthetic INSL3 itself does not display relaxin-like activity although analogue (Analogue A) with an introduced arginine residue in the B-chain giving it an Arg cassette in the exact relaxin position does possess weak activity. In order to identify further the structural features that impart relaxin function, solid phase peptide synthesis was used to prepare three additional analogues for bioassay. Each of these contained point substitutions within the arginine cassette. Analogue D contained the full human relaxin binding cassette, Analogue G consisted of the native INSL3 sequence containing an Arg to Ala substitution, and Analogue E was a further modification of Analogue A, with the same substitution. Each analogue was fully chemically characterized by a number of criteria. Detailed circular dichrosim spectroscopy analyses showed that the changes caused little alteration of secondary structure and, hence, overall conformation. However, each analogue displayed only weak relaxin-like activity. These results indicate that while the arginine cassette is vital for relaxin-like activity, there are additional, as yet unidentified structural requirements for relaxin binding.
Claasz AA, Bond CP, Bathgate RA, et al. Relaxin-like bioactivity of ovine Insulin 3 (INSL3) analogues. Eur J Biochem. 2002;269(24):6287-93.
Several orphan G protein-coupled receptors homologous to gonadotropin and thyrotropin receptors have recently been identified and named as LGR4-8. INSL3, also known as Leydig insulin-like peptide or relaxin-like factor, is a relaxin family member expressed in testis Leydig cells and ovarian theca and luteal cells. Male mice mutant for INSL3 exhibit cryptorchidism or defects in testis descent due to abnormal gubernaculum development whereas overexpression of INSL3 induces ovary descent in transgenic females. Because transgenic mice missing the LGR8 gene are also cryptorchid, INSL3 was tested as the ligand for LGR8. Here, we show that treatment with INSL3 stimulated cAMP production in cells expressing recombinant LGR8 but not LGR7. In addition, interactions between INSL3 and LGR8 were demonstrated following ligand receptor cross-linking. Northern blot analysis indicated that the LGR8 transcripts are expressed in gubernaculum whereas treatment of cultured gubernacular cells with INSL3 stimulated cAMP production and thymidine incorporation. The present study identified the ligand for an orphan G protein-coupled receptor based on common phenotypes of ligand and receptor null mice. Demonstration of INSL3 as the ligand for LGR8 facilitates understanding of the mechanism of testis descent and allows studies on the role of INSL3 in gonadal and other physiological processes.
Kumagai J, Hsu SY, Matsumi H, et al. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J Biol Chem. 2002;277(35):31283-6.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 035-27 | INSL3 / RLF (Human) | 100 µg | $521 |
| 035-27A | INSL3 / RLF (Human) | 50 µg | $405 |
| 036-78 | INSL3 / RLF (Rat) | 100 ug | $415 |
Social Network Confirmation