

The relaxin family of peptide hormones and their cognate GPCRs are becoming physiologically well-characterized in the cardiovascular system and particularly in female reproductive processes. Much less is known about the physiology and pharmacology of these peptides in male reproduction, particularly as regards humans. H2-relaxin is involved in prostate function and growth, while insulin-like peptide 3 (INSL3) is a major product of the testicular Leydig cells and, in the adult, appears to modulate steroidogenesis and germ cell survival. In the fetus, INSL3 is a key hormone expressed shortly after sex determination and is responsible for the first transabdominal phase of testicular descent. Importantly, INSL3 is becoming a very useful constitutive biomarker reflecting both fetal and post-natal development. Nothing is known about roles for INSL4 in male reproduction and only very little about relaxin-3, which is mostly considered as a brain peptide, or INSL5. The former is expressed at very low levels in the testes, but has no known physiology there, whereas the INSL5 knockout mouse does exhibit a testicular phenotype with mild effects on spermatogenesis, probably due to a disruption of glucose homeostasis. INSL6 is a major product of male germ cells, although it is relatively unexplored with regard to its physiology or pharmacology, except that in mice disruption of the INSL6 gene leads to a disruption of spermatogenesis. Clinically, relaxin analogues may be useful in the control of prostate cancer, and both relaxin and INSL3 have been considered as sperm adjuvants for in vitro fertilization.Ivell R, Agoulnik AI, Anand-ivell R. Br J Pharmacol. 2017;174(10):990-1001.
This study was designed to show whether placental relaxin (RLN), its receptor (RXFP1), or insulin-like peptide 4 (INSL4) might have altered expression in patients with placenta accreta. The baseline expression of their genes through gestation (n = 34) was quantitated in the placental basal plate (BP) and villous trophoblast (TR), and compared to their expression in placenta accreta (n = 6). The proteins were also immunolocalized and quantitated in the accreta tissues. The messenger RNAs (mRNAs) of matrix metalloproteinase 9, -2, and tissue inhibitors of matrix metalloproteinase (TIMP)-1 were also measured. Results demonstrated that the BP and TR expressed low levels of RLN/RXFP1 and INSL4 through gestation. In accreta, increased RLN gene and protein in BP were associated with antepartum bleeding whereas INSL4 expression decreased throughout the TR. There were no changes in mRNAs for MMPs, but TIMP-1 was increased only in the invasive TR.
Goh W, Yamamoto SY, Thompson KS, Bryant-greenwood GD. Reprod Sci. 2013;20(8):968-80.
OBJECTIVE: The objective of the study was to evaluate maternal serum pro-early placenta insulin like (proEPIL) levels during normal and pathologic pregnancy by using a newly developed enzyme-linked immunosorbent assay, based on a monoclonal antibody designated EPIL15 and directed to the pro-EPIL C-chain 98-108 region.STUDY DESIGN: In a group of healthy pregnant women (n = 22), proEPIL peptide serum levels were measured longitudinally throughout gestation (8-12, 20-24, and 30-34 weeks). Serum proEPIL levels were measured in women with preterm labor (n = 24), intrauterine growth restriction (n = 27), and preeclampsia (n = 12).RESULTS: In healthy pregnant women, a significant rise of serum pro-EPIL levels (mean +/- SEM) was observed during the third trimester of gestation (30.97 +/- 2.978 ng/mL; P < .01), with the highest serum levels at 30-34 weeks' gestation (P < .001). Serum proEPIL levels were found elevated in women with intrauterine growth restriction (107.4 +/- 12.99 ng/mL), preeclampsia (104.8 +/- 36.20 ng/mL), or preterm labor (183.8 +/- 36.42 ng/mL) in comparison with levels observed in healthy pregnant women (P < .001).CONCLUSION: These results showed that proEPIL secretion increases in the last trimester during normal pregnancy and is highly secreted in women with pathologic conditions.
Bruni L, Luisi S, Ferretti C, et al. Am J Obstet Gynecol. 2007;197(6):606.e1-4.
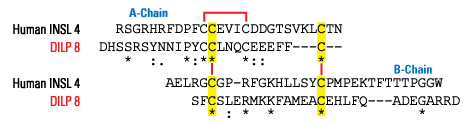
Currently, four relaxin peptide family receptors are known: LGR7 is the relaxin receptor, although it also interacts specifically with relaxin-3; LGR8 is the insulin-like factor 3 (INSL3) receptor; and GPCR135 or the somatostatin- and angiotensin-like peptide receptor (SALPR) and GPCR142 are both specific relaxin-3 receptors. Because these receptors coevolved together with their relaxin ligands, phylogenetic analysis of these sequences can provide insight into peptide-receptor interactions and even predict interacting partners for INSL4, INSL5, and INSL6, the receptors for which are unknown.Wilkinson TN, Speed TP, Tregear GW, Bathgate RA. Ann N Y Acad Sci. 2005;1041:534-9.
AIMS: Recently, we were able to show that the expression of early placenta insulin like growth factor (EPIL) is expressed by highly motile HER-2-positive breast cancer cells in vitro (Brandt et al., Cancer Res. 2002) in Paget cells in vivo and indicates a poor clinical prognosis, irrespectively of other prognostic factors.
METHODS: In order to demonstrate the interplay between HER-2 and Epil we established a cellular model for high simultaneous Epil and HER-2 expression. The HER-2-positive breast cancer cell line SKBR3 was modified with an EPIL expression vector. In addition, an assay for the knockdown of EPIL-expression via siRNA was established. Erk1/2 expression was measured via Western Blot. The phenotype of the viable cells was determined by laser scan microscopy.
RESULTS: Epil overexpression in SKBR3 cells resulted in fast and frequent protrusion formation of the cells shown by laser scan microscopy. The cells were further characterized by a significantly increased invasiveness, which could be reversed by Epil specific siRNA treatment. Increased invasiveness and morphological changes were associated with a decreased erk1/2 phosphorylation.
CONCLUSIONS: These data further supports the assumption that EPIL might provide an autocrine loop in HER-2-positive breast cancer cells that enforce metastasis, conceivably escape from adjuvant therapy and in consequence poor clinical outcome. A tight interaction between HER-2 and EPIL in invasive breast cancer cells is therefore likely. The exact mechanims remain to be elucidated.
Bürger H, Kemming D, Helms M, et al. Verh Dtsch Ges Pathol. 2005;89:201-6.
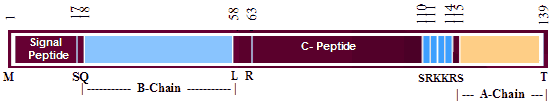
Insulin and insulin-like growth factors belong to a family of polypeptides involved in essential physiological processes. Placentin, a new member of the insulin family, was recently identified as a 139-amino acid open reading frame from a cDNA clone isolated from a subtracted library of first trimester human placenta. Tris/Tricine/SDS-polyacrylamide gel electrophoresis and immunoblot analyses of histidine-tagged recombinant placentin indicate that it is composed of two peptide chains of apparent molecular masses of 4 and 13 kDa. Conditioned media produced by recombinant expression of placentin cDNA in the placental 3AsubE cell line were assayed for biological activity and found to stimulate tyrosine phosphorylation and DNA synthesis. While these effects closely mimicked those of insulin, they were not mediated by the insulin receptor as shown by the lack of tyrosine phosphorylation of this receptor upon placentin treatment. Moreover, in cytotrophoblast primary culture, production of chorionic gonadotropin, a marker of trophoblast differentiation, was increased upon treatment with placentin-conditioned media, while unaffected by insulin. These results suggest that placentin might participate in the cellular proliferation and/or differentiation processes during placental development.
Koman A, Cazaubon S, Couraud PO, Ullrich A, Strosberg AD. J Biol Chem. 1996;271(34):20238-41.
A new member of the insulin gene superfamily was identified by screening a subtracted cDNA library of first-trimester human placenta and, hence, was tentatively named early placenta insulin-like peptide (EPIL). In this paper, we report the cloning and sequencing of the EPIL cDNA and the EPIL gene (INSL4). Comparison of the deduced amino acid sequence of the early placenta insulin-like peptide revealed significant overall and structural homologies with members of the insulin-like hormone superfamily. Moreover, the organization of the early placenta insulin-like gene, which is composed of two exons and one intron, is similar to that of insulin and relaxin. By in situ hybridization, the INSL4 gene was assigned to band p24 of the short arm of chromosome 9. RT-PCR analysis of EPIL tissue distribution revealed that its transcripts are expressed in the placenta and uterus.
Chassin D, Laurent A, Janneau JL, Berger R, Bellet D. Genomics. 1995;29(2):465-70.
| Type of samples
|
Measured Concentration (Mean ± S.D.) after using ½ X diluted sample in assay
|
|
Pregnant Women Serum (in-house samples N=26) |
28.09 ± 2.84 (ng/ml) |
|
Normal Human Serum (in-house samples N=7) |
17.85 ± 2.55 (ng/ml) |
|
Cord Blood (in-house samples N=21) |
7.85 ± 0.918 (ng/ml) |
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 035-33 | INSL4 / EPIL (Human) | 100 µg | $470 |
| H-035-33 | INSL4 / EPIL (Human) - Antibody | 50 µl | $571 |
| T-035-33 | INSL4 / EPIL (Human) - I-125 Labeled | 10 µCi | $1322 |
| 035-31 | INSL4 A-Chain (Human) | 100 µg | $267 |
| 035-32 | INSL4 B-Chain (Human) | 100 µg | $336 |
| 035-56 | prepro-INSL4 C-Peptide (64-110) (Human) | 100 µg | $377 |
| H-035-56 | prepro-INSL4 C-Peptide (64-110) (Human) - Antibody | 100 µl | $377 |
| EK-035-56 | prepro-INSL4 C-Peptide (64-110) (Human) - EIA kit | 96 wells | $610 |
| G-035-56 | prepro-INSL4 C-Peptide (64-110) (Human) - Purified IgG Antibody | 100 µg | $377 |
| 036-53 | INSL4, short B-Chain (Human) | 100 µg | $428 |
Social Network Confirmation