|
|
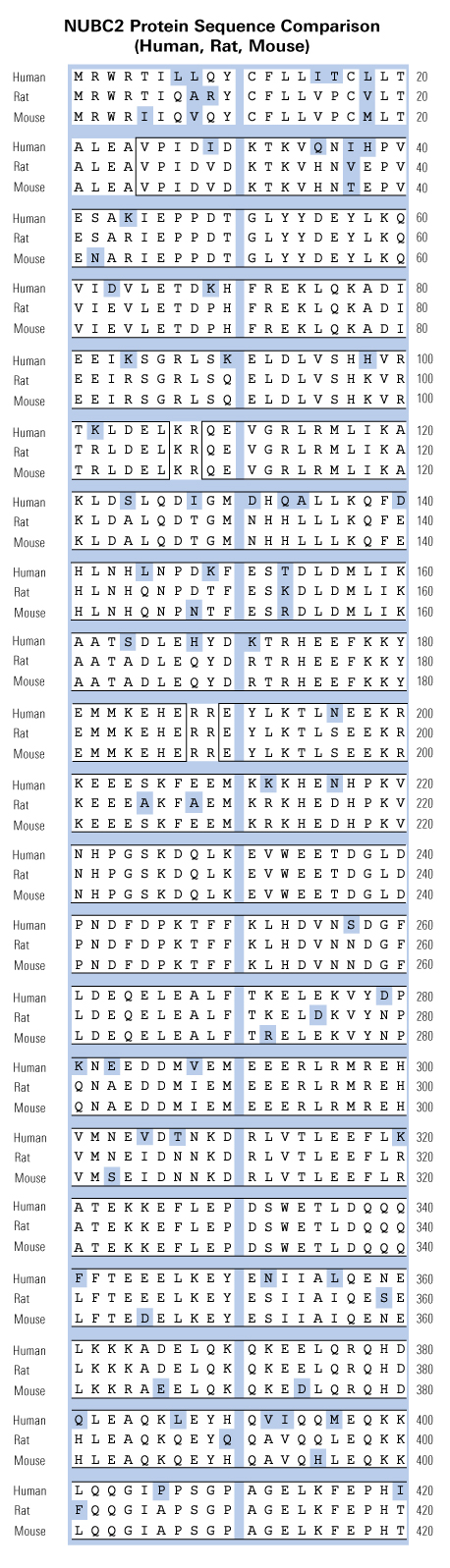 |
BACKGROUND/AIMS: Nesfatin-1, processed from nucleobindin-2 (NUCB2), is a potent anorexigenic peptide being expressed in rodent hypothalamic nuclei and involved in the regulation of feeding behavior and body weight in animals. The present study aimed to investigate NUCB2/nesfatin-1 protein expression in the human hypothalamus as well as its correlation with body weight.METHODS: Sections of hypothalamus and adjacent cholinergic basal forebrain nuclei, including the nucleus basalis of Meynert (NBM) and the diagonal band of Broca (DBB), from 25 autopsy cases (17 males, 8 females; 8 lean, 9 overweight, 8 obese) were examined using immunohistochemistry and double immunofluorescence labeling.RESULTS: Prominent NUCB2/nesfatin-1 immunoexpression was detected in supraoptic, paraventricular, and infundibular nuclei, lateral hypothalamic area (LHA)/perifornical region, and NBM/DBB. NUCB2/nesfatin-1 was found to extensively colocalize with (a) oxytocin and vasopressin in paraventricular and supraoptic nuclei, (b) melanin-concentrating hormone in the LHA, and (c) cocaine- and amphetamine-regulated transcript in infundibular and paraventricular nuclei and LHA. Interestingly, in the LHA, NUCB2/nesfatin-1 protein expression was significantly decreased in obese, compared with lean (p < 0.01) and overweight (p < 0.05) subjects.CONCLUSIONS: The findings of the present study are suggestive of a potential role for NUCB2/nesfatin-1 as an integral regulator of food intake and energy homeostasis in the human hypothalamus. In the LHA, an appetite- and reward-related brain area, reduced NUCB2/nesfatin-1 immunoexpression may contribute to dysregulation of homeostatic and/or hedonic feeding behavior and obesity. NUCB2/nesfatin-1 localization in NBM/DBB might imply its participation in the neuronal circuitry controlling cognitive influences on food intake and give impetus towards unraveling additional biological actions of NUCB2/nesfatin-1 in human neuronal networks.This publication used the nesfatin-1 antibody (H-003-22) from Phoenix Pharmaceuticals.
Psilopanagioti A, Nikou S, Papadaki H. Nucleobindin-2/Nesfatin-1 in the Human Hypothalamus Is Reduced in Obese Subjects and Colocalizes with Oxytocin, Vasopressin, Melanin-Concentrating Hormone, and Cocaine- and Amphetamine-Regulated Transcript. Neuroendocrinology. 2019;108(3):190-200.
We discovered a new anorexigenic protein, nesfatin/nucleobindin-2 (NUCB2), which includes an EF-hand, calcium-binding motif. Nesfatin/NUCB2 is converted to nesfatin-1, which may be a physiologically active form in the body. Centrally and systemically administered nesfatin-1 inhibits appetite and body weight gain in rodents. The mid-segment of nesfatin-1 appears to be important in the inhibition of food intake. Intranasal administration of the mid-segment inhibits appetite. Nesfatin-1 may also be involved in the regulation of gastrointestinal function and insulin secretion. We have summarized the recent progress in the research of nesfatin-1.
Shimizu H, Mori M. Nesfatin-1: its role in the diagnosis and treatment of obesity and some psychiatric disorders. Methods Mol Biol. 2013;963:327-38.
Nesfatin-1, a satiety-inducing peptide identified in hypothalamic regions that regulate energy balance, is an integral regulator of energy homeostasis and a putative glucose-dependent insulin coadjuvant. We investigated its production by human cardiomyocytes and its effects on glucose uptake, in the main cardiac glucose transporter GLUT-4 and in intracellular signaling. Quantitative RT-PCR, Western blots, confocal immunofluorescence microscopy, and ELISA of human and murine cardiomyocytes and/or cardiac tissue showed that cardiomyocytes can synthesize and secrete nesfatin-1. Confocal microscopy of cultured cardiomyocytes after GLUT-4 labeling showed that nesfatin-1 mobilizes this glucose transporter to cell peripherals. The rate of 2-deoxy-D-[(3)H]glucose incorporation demonstrated that nesfatin-1 induces glucose uptake by HL-1 cells and cultured cardiomyocytes. Nesfatin-1 induced dose- and time-dependent increases in the phosphorylation of ERK1/2, AKT, and AS160. In murine and human cardiac tissue, nesfatin-1 levels varied with diet and coronary health. In conclusion, human and murine cardiomyocytes can synthesize and secrete nesfatin-1, which is able to induce glucose uptake and the mobilization of the glucose transporter GLUT-4 in these cells. Nesfatin-1 cardiac levels are regulated by diet and coronary health.
Feijóo-bandín S, Rodríguez-penas D, García-rúa V, et al. Nesfatin-1 in human and murine cardiomyocytes: synthesis, secretion, and mobilization of GLUT-4. Endocrinology. 2013;154(12):4757-67.
Hunger and satiety are regulated in a complex fashion by a few food intake stimulatory (orexigenic) and a multitude of inhibitory (anorexigenic) factors produced in the periphery (mainly in the gastrointestinal tract) or directly in the brain. Within the brain, the hypothalamus plays a pivotal role as a production site of food intake regulatory factors. Importantly, this site integrates peripheral and central signaling factors to orchestrate food intake and in the long term body weight. Our knowledge on these regulatory pathways is not static but rather rapidly changing as new factors as well as up- and downstream signaling pathways of already known transmitters are uncovered. Hypothalamic nucleobindin2 (NUCB2), the precursor of nesfatin-1, was first described in 2006 and nesfatin-1 found to be a novel anorexigenic modulator of food intake and body weight. The initial report stimulated several groups to investigate the biological actions of nesfatin-1 and subsequent studies delineated the underlying brain mechanisms involved in its food reducing effect. Of interest was the demonstration that NUCB2 also exerts its anorexigenic action in the paraventricular nucleus of the hypothalamus and is regulated at this site by changes in metabolic status with a diurnal rhythm inversely related to that of feeding in rats. The present review describes the current state-of-knowledge on central nesfatin-1's effects on food intake and body weight and highlights important missing links regarding cellular signaling mechanisms involved in nesfatin-1's action.
Stengel A, Taché Y. Role of NUCB2/Nesfatin-1 in the hypothalamic control of energy homeostasis. Horm Metab Res. 2013;45(13):975-9.
AIMS/HYPOTHESIS: The actions of peripherally administered nesfatin-1 on glucose homeostasis remain controversial. The aim of this study was to characterize the mechanisms by which peripheral nesfatin-1 regulates glucose metabolism.
METHODS: The effects of nesfatin-1 on glucose metabolism were examined in mice by continuous infusion of the peptide via osmotic pumps. Changes in AKT phosphorylation and Glut4 were investigated by Western blotting and immnuofluorescent staining. Primary myocytes, adipocytes and hepatocytes were isolated from male mice.
RESULTS: Continuous peripheral infusion of nesfatin-1 altered glucose tolerance and insulin sensitivity in mice fed either normal or high fat diet, while central administration of nesfatin-1 demonstrated no effect. Nesfatin-1 increases insulin secretion in vivo, and in vitro in cultured min6 cells. In addition, nesfatin-1 up-regulates the phosphorylation of AKT in pancreas and min6 islet cells. In mice fed normal diet, peripheral nesfatin-1 significantly increased insulin-stimulated phosphorylation of AKT in skeletal muscle, adipose tissue and liver; similar effects were observed in skeletal muscle and adipose tissue in mice fed high fat diet. At basal conditions and after insulin stimulation, peripheral nesfatin-1 markedly increased GLUT4 membrane translocation in skeletal muscle and adipose tissue in mice fed either diet. In vitro studies showed that nesfatin-1 increased both basal and insulin-stimulated levels of AKT phosphorylation in cells derived from skeletal muscle, adipose tissue and liver.
CONCLUSIONS: Our studies demonstrate that nesfatin-1 alters glucose metabolism by mechanisms which increase insulin secretion and insulin sensitivity via altering AKT phosphorylation and GLUT 4 membrane translocation in the skeletal muscle, adipose tissue and liver.
Li Z, Gao L, Tang H, et al. Peripheral effects of nesfatin-1 on glucose homeostasis. PLoS ONE. 2013;8(8):e71513.
Nesfatin-1 is a novel anorexigenic hormone which has close relationship with diabetes, obese, anorexia nervosa, psychiatric disorders and neurogenic diseases. The aim of our study was to evaluate levels of plasma nesfatin-1 among patients presenting with coronary artery disease and the correlation between nesfatin-1 levels and other clinical parameters. Fasting plasma levels of nesfatin-1 were tested in 48 acute myocardial infarction (AMI) patients, 74 stable angina pectoris (SAP) patients and 34 control subjects. All of them were examined by coronary angiography. The severity of coronary atherosclerosis was assessed using the Gensini score. Plasma nesfatin-1 levels were significantly lower in AMI group than SAP group or control group (0.91±0.08ng/mL vs. 0.98±0.19ng/mL and 1.09±0.39ng/mL, respectively, P<0.05). In AMI patients, plasma nesfatin-1 levels were negatively correlated with high-sensitivity C-reactive protein, neutrophil% or Gensini scores. Such information implies that lower nesfatin-1 concentration may play a very important role in the development of AMI.
Dai H, Li X, He T, et al. Decreased plasma nesfatin-1 levels in patients with acute myocardial infarction. Peptides. 2013;46:167-71.
Nesfatin-1, discovered by Oh-I and his coworkers in 2006, is a multi-functional peptide hormone with an approximate MW of 9.8 kDa and a half-life of 23.5 minutes. This peptide is found in three different forms, nesfatin-1, nesfatin-2 and nesfatin-3, all three of which are formed from the precursor NUCB2 by proteolytic processing. The 30-amino acid middle segment of nesfatin-1 (M30) is responsible for limiting food intake, while the exact physiological role of nesfatin-2 and nesfatin-3 are unknown. This review will focus on nesfatin-1 in relation with tissue and fluid distribution, considerations for its analysis in body fluids, and its potential as a biomarker for some diseases.
Aydin S. Role of NUCB2/nesfatin-1 as a possible biomarker. Curr Pharm Des. 2013;19(39):6986-92.
Nesfatin-1, derived from an 82-amino-acid peptide precursor protein, nucleobindin-2 (NUCB2) is a highly conserved peptide across mammalian species. Initial functional and neuroanatomical studies on NUCB2/nesfatin-1 in the central nervous system have supported a role for NUCB2/nesfatin-1 as a novel satiety molecule. In recent years, however, it has become apparent that this neuropeptide is involved in various other processes, one of which is the stress response. Stress-associated activation of NUCB2/nesfatin-1 neurons, together with nesfatin-1's central actions in the brain is indicative of its significance in the stress adaptation response. Interestingly, increasing body of evidence implicates also NUCB2/nesfatin-1 in various forms of stress-associated psychopathologies, such as anxiety and depression. In this review, we will outline evidence that has significantly broadened our understanding of the biological significance of NUCB2/nesfatin-1 far beyond to be only a hypothalamic peptide with potent anorexigenic actions. NUCB2/nesfatin-1 neurons in the brain seem to emerge as novel, integral regulators of the stress adaptation response.
Emmerzaal TL, Kozicz T. Nesfatin-1; implication in stress and stress-associated anxiety and depression. Curr Pharm Des. 2013;19(39):6941-8.
NUCB2 (1-83) has been recently reported as an anorexigenic and anti-hyperglycemic peptide. Here we report that NUCB2 (1-83) promotes osteogenesis. It was found after two months of once-a-day intravenous injection of NUCB2 (1-83), bone mineral density of femora and lumbar vertebrae were increased in ovariectomized rats. NUCB2 (1-83) also increased the alkaline phosphatase activity and promoted mineralization in mouse MC3T3-E1 preosteoblastic cell line. When either both Arg0 and Arg3 or Ser2 were mutated to Ala, the pro-osteogenic activity was completely lost, indicating that these residues are structurally important for its biological function. Furthermore, it encumbered osteoclastic differentiation of RAW 264.7 macrophage. It also excluded any possibility of the effect caused by contaminants or experimental faults, and demonstrated that the pro-osteogenic activity observed was a specific effect of NUCB2 itself. These findings warranted that further studies on NUCB2 would be valuable for the treatment of bone metabolic diseases especially for osteoporosis.
Li R, Wu Q, Zhao Y, et al. The novel pro-osteogenic activity of NUCB2(1-83.). PLoS ONE. 2013;8(4):e61619.
STUDY OBJECTIVES: Millions suffer from sleep disorders that often accompany severe illnesses such as major depression; a leading psychiatric disorder characterized by appetite and rapid eye movement sleep (REMS) abnormalities. Melanin-concentrating hormone (MCH) and nesfatin-1/NUCB2 (nesfatin) are strongly co - expressed in the hypothalamus and are involved both in food intake regulation and depression. Since MCH was recognized earlier as a hypnogenic factor, we analyzed the potential role of nesfatin on vigilance.
DESIGN: We subjected rats to a 72 h-long REMS deprivation using the classic flower pot method, followed by a 3 h-long 'rebound sleep'. Nesfatin mRNA and protein expressions as well as neuronal activity (Fos) were measured by quantitative in situ hybridization technique, ELISA and immunohistochemistry, respectively, in 'deprived' and 'rebound' groups, relative to controls sacrificed at the same time. We also analyzed electroencephalogram of rats treated by intracerebroventricularly administered nesfatin-1, or saline.
RESULTS: REMS deprivation downregulated the expression of nesfatin (mRNA and protein), however, enhanced REMS during 'rebound' reversed this to control levels. Additionally, increased transcriptional activity (Fos) was demonstrated in nesfatin neurons during 'rebound'. Centrally administered nesfatin-1 at light on reduced REMS and intermediate stage of sleep, while increased passive wake for several hours and also caused a short-term increase in light slow wave sleep. CONCLUSIONS: The data designate nesfatin as a potential new factor in sleep regulation, which fact can also be relevant in the better understanding of the role of nesfatin in the pathomechanism of depression.
Vas S, Ádori C, Könczöl K, et al. Nesfatin-1/NUCB2 as a potential new element of sleep regulation in rats. PLoS ONE. 2013;8(4):e59809.
The recently discovered Nesfatin-1 plays a role in appetite regulation as a satiety factor through hypothalamic leptin-independent mechanisms. Nesfatin-1 is co-expressed with Melanin-Concentrating Hormone (MCH) in neurons from the tuberal hypothalamic area (THA) which are recruited during sleep states, especially paradoxical sleep (PS). To help decipher the contribution of this contingent of THA neurons to sleep regulatory mechanisms, we thus investigated in rats whether the co-factor Nesfatin-1 is also endowed with sleep-modulating properties. Here, we found that the disruption of the brain Nesfatin-1 signaling achieved by icv administration of Nesfatin-1 antiserum or antisense against the nucleobindin2 (NUCB2) prohormone suppressed PS with little, if any alteration of slow wave sleep (SWS). Further, the infusion of Nesfatin-1 antiserum after a selective PS deprivation, designed for elevating PS needs, severely prevented the ensuing expected PS recovery. Strengthening these pharmacological data, we finally demonstrated by using c-Fos as an index of neuronal activation that the recruitment of Nesfatin-1-immunoreactive neurons within THA is positively correlated to PS but not to SWS amounts experienced by rats prior to sacrifice. In conclusion, this work supports a functional contribution of the Nesfatin-1 signaling, operated by THA neurons, to PS regulatory mechanisms. We propose that these neurons, likely releasing MCH as a synergistic factor, constitute an appropriate lever by which the hypothalamus may integrate endogenous signals to adapt the ultradian rhythm and maintenance of PS in a manner dictated by homeostatic needs. This could be done through the inhibition of downstream targets comprised primarily of the local hypothalamic wake-active orexin- and histamine-containing neurons.
Jego S, Salvert D, Renouard L, et al. Tuberal hypothalamic neurons secreting the satiety molecule Nesfatin-1 are critically involved in paradoxical (REM) sleep homeostasis. PLoS ONE. 2012;7(12):e52525.
Nesfatin-1 is an 82 amino acid N-terminal fragment of nucleobindin2 that was consistently shown to reduce dark phase food intake upon brain injection in rodents. We recently reported that nesfatin-1(1-82) injected intracerebroventricularly (icv) reduces dark phase feeding in mice. Moreover, intraperitoneal injection of mid-fragment nesfatin-1 (nesfatin-1(30-59)) mimics the food intake-reducing effects of nesfatin-1(1-82), whereas N-terminal (nesfatin-1(1-29)) and C-terminal fragments (nesfatin-1(60-82)) did not. We therefore characterized the structure-activity relationship of nesfatin-1 injected icv to influence the dark phase meal pattern in mice. Mouse nesfatin-1(1-29), nesfatin-1(30-59), nesfatin-1(60-82) or vehicle was injected icv in freely fed C57Bl/6 mice immediately before the dark phase and food intake was monitored using an automated episodic feeding monitoring system. Nesfatin-1(30-59) (0.1, 0.3, 0.9 nmol/mouse) induced a dose-related reduction of 4-h food intake by 28%, 49% and 49% respectively resulting in a 23% decreased cumulative 24-h food intake compared to vehicle at the 0.3 nmol/mouse dose (p<0.05). The peak reduction occurred during the 3rd (-96%) and 4th hour (-91%) post injection and was associated with a reduced meal frequency (0-4h: -47%) and prolonged inter-meal intervals (3.1-times) compared to vehicle (p<0.05), whereas meal size was not altered. In contrast, neither nesfatin-1(1-29) nor nesfatin-1(60-82) reduced dark phase food intake at equimolar doses although nesfatin-1(60-82) prolonged inter-meal intervals (1.7-times, p<0.05). Nesfatin-1(30-59) is the active core of nesfatin-1(1-82) to induce satiety indicated by a reduced meal number during the first 4h post injection. The delayed onset may be indicative of time required to modulate other hypothalamic and medullary networks regulating nocturnal feeding as established for nesfatin-1.
Stengel A, Goebel-stengel M, Wang L, Kato I, Mori M, Taché Y. Nesfatin-1(30-59) but not the N- and C-terminal fragments, nesfatin-1(1-29) and nesfatin-1(60-82) injected intracerebroventricularly decreases dark phase food intake by increasing inter-meal intervals in mice. Peptides. 2012;35(2):143-8.
Nesfatin-1 is a recently discovered anorexigen, and we first reported nesfatin-like immunoreactivity in the pancreatic β-cells. The aim of this study was to characterize the effects of nesfatin-1 on whole-body energy homeostasis, insulin secretion, and glycemia. The in vivo effects of continuous peripheral delivery of nesfatin-1 using osmotic minipumps on food intake and substrate partitioning were examined in ad libitum-fed male Fischer 344 rats. The effects of nesfatin-1 on glucose-stimulated insulin secretion (GSIS) were examined in isolated pancreatic islets. L6 skeletal muscle cells and isolated rat adipocytes were used to assess the effects of nesfatin-1 on basal and insulin-mediated glucose uptake as well as on major steps of insulin signaling in these cells. Nesfatin-1 reduced cumulative food intake and increased spontaneous physical activity, whole-body fat oxidation, and carnitine palmitoyltransferase I mRNA expression in brown adipose tissue but did not affect uncoupling protein 1 mRNA in the brown adipose tissue. Nesfatin-1 significantly enhanced GSIS in vivo during an oral glucose tolerance test and improved insulin sensitivity. Although insulin-stimulated glucose uptake in L6 muscle cells was inhibited by nesfatin-1 pretreatment, basal and insulin-induced glucose uptake in adipocytes from nesfatin-1-treated rats was significantly increased. In agreement with our in vivo results, nesfatin-1 enhanced GSIS from isolated pancreatic islets at both normal (5.6 mm) and high (16.7 mm), but not at low (2 mm), glucose concentrations. Furthermore, nesfatin-1/nucleobindin 2 release from rat pancreatic islets was stimulated by glucose. Collectively, our data indicate that glucose-responsive nesfatin-1 regulates insulin secretion, glucose homeostasis, and whole-body energy balance in rats.
Gonzalez R, Perry RL, Gao X, et al. Nutrient responsive nesfatin-1 regulates energy balance and induces glucose-stimulated insulin secretion in rats. Endocrinology. 2011;152(10):3628-37.
Nesfatin-1 is a recently identified anorexigenic peptide derived from its precursor protein, nonesterified fatty acid/nucleobindin 2 (NUCB2). Although the hypothalamus is pivotal for the maintenance of energy homeostasis, adipose tissue plays an important role in the integration of metabolic activity and energy balance by communicating with peripheral organs and the brain via adipokines. Currently no data exist on nesfatin-1 expression, regulation, and secretion in adipose tissue. We therefore investigated NUCB2/nesfatin-1 gene and protein expression in human and murine adipose tissue depots. Additionally, the effects of insulin, dexamethasone, and inflammatory cytokines and the impact of food deprivation and obesity on nesfatin-1 expression were studied by quantitative RT-PCR and Western blotting. We present data showing NUCB2 mRNA (P < 0.001), nesfatin-1 intracellular protein (P < 0.001), and secretion (P < 0.01) were significantly higher in sc adipose tissue compared with other depots. Also, nesfatin-1 protein expression was significantly increased in high-fat-fed mice (P < 0.01) and reduced under food deprivation (P < 0.01) compared with controls. Stimulation of sc adipose tissue explants with inflammatory cytokines (TNFalpha and IL-6), insulin, and dexamethasone resulted in a marked increase in intracellular nesfatin-1 levels. Furthermore, we present evidence that the secretion of nesfatin-1 into the culture media was dramatically increased during the differentiation of 3T3-L1 preadipocytes into adipocytes (P < 0.001) and after treatments with TNF-alpha, IL-6, insulin, and dexamethasone (P < 0.01). In addition, circulating nesfatin-1 levels were higher in high-fat-fed mice (P < 0.05) and showed positive correlation with body mass index in human. We report that nesfatin-1 is a novel depot specific adipokine preferentially produced by sc tissue, with obesity- and food deprivation-regulated expression.
Ramanjaneya M, Chen J, Brown JE, et al. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology. 2010;151(7):3169-80.
Numerous peptides released from endocrine cells in the intestinal mucosa were established early on to be involved in the physiological regulation of food intake with a prominent role in termination of food ingestion when nutrients pass along the intestinal tract. Recently, peptides released from X/A-like endocrine cells of the gastric oxyntic mucosa were recognized as additional key players in the regulation of feeding and energy expenditure. Gastric X/A-like cells release the octanoylated peptide, ghrelin, the only known peripherally produced hormone stimulating food intake through interaction with growth hormone secretagogue 1a receptor (GHS-R1a). Additionally, non-octanoylated (des-acyl) ghrelin present in the circulation at higher levels than ghrelin is currently discussed as potential modulator of food intake by opposing ghrelin's action independent from GHS-R1a although the functional significance remains to be established. Obestatin, a ghrelin-associated peptide was initially reported as anorexigenic modulator of ghrelin's orexigenic action. However, subsequent reports did not support this contention. Interesting is the recent identification of nesfatin-1, a peptide derived from the nucleobindin2 gene prominently expressed in gastric X/A-like cells in different vesicles than ghrelin. Circulating nesfatin-1 levels vary with metabolic state and peripheral or central injection inhibits dark phase feeding in rodents. Overall, these data point to an important role of gastric X/A-like cells in food intake regulation through the expression of the orexigenic peptide ghrelin along with des-acyl ghrelin and nesfatin-1 capable of reducing food intake upon exogenous injection although their mechanisms of action and functional significance remain to be established.
Stengel A, Goebel M, Wang L, Taché Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides. 2010;31(2):357-69.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 003-22A | Nesfatin-1 (1-82) (Rat) | 20 µg | $203 |
| 003-22B | Nesfatin-1 (1-82) (Rat) | 100 µg | $470 |
| H-003-22 | Nesfatin-1 (1-82) (Rat) - Antibody | 100 µl | $287 |
| EK-003-22 | Nesfatin-1 (1-82) (Rat) - EIA Kit, extraction-free | 96 wells | $668 |
| 003-26 | Nesfatin-1 (1-82) (Human) | 20 µg | $202 |
| 003-26B | Nesfatin-1 (1-82) (Human) | 100 µg | $470 |
| 009-76 | Nesfatin-1 (1-29) (Mouse) | 100 µg | $317 |
| 003-24 | Nesfatin-1 (1-45) / Nesfatin-1, N-terminal (Human) | 100 µg | $299 |
| H-003-24 | Nesfatin-1 (1-45) / Nesfatin-1, N-terminal (Human) - Antibody | 100 µl | $635 |
| B-003-24 | Nesfatin-1 (1-45) / Nesfatin-1, N-terminal (Human) - Biotin Labeled | 10 µg | $444 |
Social Network Confirmation