Neurosecretory protein GL (NPGL) and neurosecretory protein GM (NPGM) are paralogs recently discovered in birds and in mammals. The post-translational products of NPGL and of NPGM genes include a signal peptidesequence, a glycine amidation signal, and a dibasic amino acid cleavage site. This suggests that the mature forms of NPGL and of NPGM are small proteins secreted in the hypothalamus and containing an amidated C-terminus. However, endogenous NPGL and NPGM have not yet been identified. Chicken NPGL and NPGM have two highly conserved Cys residues that are likely to form a disulfide bond, while mammalian NPGM has one additional Cys residue located between the two conserved Cys residues and the correct disulfide bond pattern is unclear. In this study, we prepared rat NPGM to elucidate the structure of its mature form. We first expressed the predicted mature NPGM, containing an extra C-terminal Gly, in Escherichia coli SHuffle cells, which are engineered to promote the formation of native disulfide bridges in recombinant proteins. We observed the presence of a disulfide bond between the N-terminal Cys residue and the second Cys residue, while the C-terminal Cys residue was free. Secondly, we transfected a construct containing the entire NPGM open reading frame into Chinese Hamster Ovary cells, and observed that NPGM was cleaved immediately after the signal peptide and that it was secreted into the medium. Furthermore, the protein presented a disulfide bond at the same location observed in recombinant NPGM.
Masuda K, Furumitsu M, Taniuchi S, Iwakoshi-ukena E, Ukena K. FEBS Open Bio. 2015;5:844-51.
To find novel neuropeptide and/or peptide hormone precursors in the avian brain, we performed a cDNA subtractive screen of the chicken hypothalamic infundibulum, which contains one of the feeding and neuroendocrine centers. After sequencing 596 clones, we identified a novel cDNA encoding a previously unknown protein. The deduced precursor protein consisted of 182 amino acid residues, including one putative small secretory protein of 80 amino acid residues. This small protein was flanked at the N-terminus by a signal peptide and at the C-terminus by a glycine amidation signal and a dibasic amino acid cleavage site. Because the predicted C-terminal amino acids of the small protein were Gly-Leu-NH2, the small protein was named neurosecretory protein GL (NPGL). Quantitative RT-PCR analysis demonstrated specific expression of the NPGL precursor mRNA in the hypothalamic infundibulum. Furthermore, the mRNA levels in the hypothalamic infundibulum increased during post-hatching development. In situ hybridization analysis showed that the cells containing the NPGL precursor mRNA were localized in the medial mammillary nucleus and infundibular nucleus within the hypothalamic infundibulum of 8- and 15-day-old chicks. Subcutaneous infusion of NPGL in chicks increased body weight gain without affecting food intake. To our knowledge, this is the first report to describe the identification and localization of the NPGL precursor mRNA and the function of its translated product in animals. Our findings indicate that NPGL may participate in the growth process in chicks
Ukena K, Iwakoshi-ukena E, Taniuchi S, et al. Biochem Biophys Res Commun. 2014;446(1):298-303.

| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
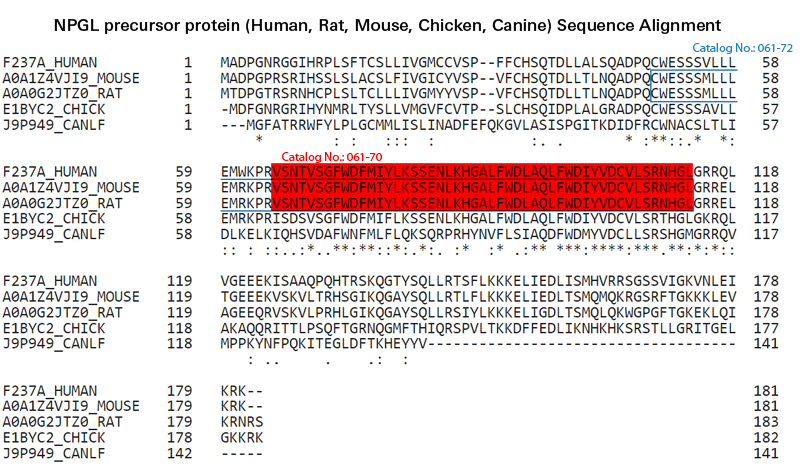
| G-061-70 | FAM237A / NPGL (65-113)-amide (Human, Rat, Mouse) - Purified IgG Antibody | 200 μg | $698 |
| H-061-70 | FAM237A / NPGL (65-113)-amide (Human, Rat, Mouse) Antibody | 50 µl | $571 |
| 061-72 | Neurosecretory Protein GL (NPGL) / FAM237A (49-113) amide (Mouse) | 100 µg | $444 |
| 061-70 | Neurosecretory Protein GL (NPGL) / FAM237A (65-113) amide (Human, Rat, Mouse) | 100 µg | $382 |
Social Network Confirmation