
Multidrug and toxic compound extrusion (MATE) family transporters are conserved in the three primary domains of life (Archaea, Bacteria and Eukarya), and export xenobiotics using an electrochemical gradient of H(+) or Na(+) across the membrane. MATE transporters confer multidrug resistance to bacterial pathogens and cancer cells, thus causing critical reductions in the therapeutic efficacies of antibiotics and anti-cancer drugs, respectively. Therefore, the development of MATE inhibitors has long been awaited in the field of clinical medicine. Here we present the crystal structures of the H(+)-driven MATE transporter from Pyrococcus furiosus in two distinct apo-form conformations, and in complexes with a derivative of the antibacterial drug norfloxacin and three in vitro selected thioether-macrocyclic peptides, at 2.1-3.0 Å resolutions. The structures, combined with functional analyses, show that the protonation of Asp 41 on the amino (N)-terminal lobe induces the bending of TM1, which in turn collapses the N-lobe cavity, thereby extruding the substrate drug to the extracellular space. Moreover, the macrocyclic peptides bind the central cleft in distinct manners, which correlate with their inhibitory activities. The strongest inhibitory peptide that occupies the N-lobe cavity may pave the way towards the development of efficient inhibitors against MATE transporters.
Tanaka Y, Hipolito CJ, Maturana AD, et al, Nature. 2013 Apr 11;496(7444):247-51. doi: 10.1038/nature12014. Epub 2013 Mar 27.
The MATE (Multidrug And Toxic Compound Extrusion) family is the most recently categorized one among five multidrug efflux transporter families. As far as we know, about twenty MATE transporters have been characterized so far. According to the information in sequence databases, huge numbers of MATE transporters seem to be present in various microorganisms. In this review, we would like to summarize the properties of the MATE-family transporters.
Kuroda T, Tsuchiya T. et al, Biochim Biophys Acta. 2009 May;1794(5):763-8. doi: 10.1016/j.bbapap.2008.11.012. Epub 2008 Dec 6.
Mammalian multidrug and toxic compound extrusion (MATE) proteins are classified into three subfamilies: classes I, II, and III. We previously showed that two of these families act as polyspecific H(+)-coupled transporters of organic cations (OCs) at final excretion steps in liver and kidney (Otsuka et al. Proc Natl Acad Sci USA 102: 17923-17928, 2005; Omote et al. Trends Pharmacol Sci 27: 587-593, 2006). Rodent MATE2 proteins are class III MATE transporters, the molecular nature, as well as transport properties, of which remain to be characterized. In the present study, we investigated the transport properties and localization of mouse MATE2 (mMATE2). On expression in human embryonic kidney (HEK)-293 cells, mMATE2 localized to the intracellular organelles and plasma membrane. mMATE2 mediated pH-dependent TEA transport with substrate specificity similar to, but distinct from, that of mMATE1, which prefers N-methylnicotinamide and guanidine as substrates. mMATE2 expressed in insect cells was solubilized and reconstituted with bacterial H(+)-ATPase into liposomes. The resultant proteoliposomes exhibited ATP-dependent uptake of TEA that was sensitive to carbonyl cyanide 3-chlorophenylhydrazone but unaffected by valinomycin in the presence of K(+). Immunologic techniques using specific antibodies revealed that mMATE2 was specifically expressed in testicular Leydig cells. Thus mMATE2 appears to act as a polyspecific H(+)/OC exporter in Leydig cells. It is concluded that all classes of mammalian MATE proteins act as polyspecific and electroneutral transporters of organic cations.
Hiasa M, et al, Am J Physiol Cell Physiol. 2007 Nov;293(5):C1437-44. Epub 2007 Aug 22.
Multidrug and toxic compound extrusion (MATE) proteins, comprising the most recently designated family of multidrug transporter proteins, are widely distributed in all kingdoms of living organisms, although their function is far from understood. The bacterial MATE-type transporters that have been characterized function as exporters of cationic drugs, such as norfloxacin and ethidium, through H(+) or Na(+) exchange. Plant MATE-type transporters are involved in the detoxification of secondary metabolites, including alkaloids. Mammalian MATE-type transporters are responsible for the final step in the excretion of metabolic waste and xenobiotic organic cations in the kidney and liver through electroneutral exchange of H(+). Thus, we propose that members of the MATE family are organic cation exporters that excrete metabolic or xenobiotic organic cations from the body.
Omote H, et al, Trends Pharmacol Sci. 2006 Nov;27(11):587-93. Epub 2006 Sep 25.
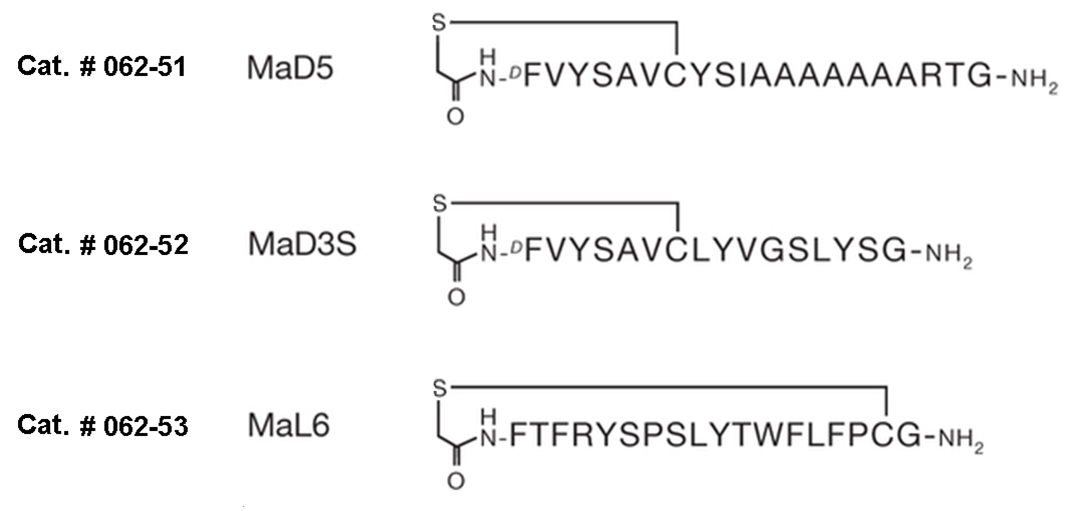
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 062-52 | MaD3S | 100 µg | $279 |
| 062-51 | MaD5 | 100 µg | $317 |
| 062-53 | MaL6 | 100 µg | $317 |
Social Network Confirmation