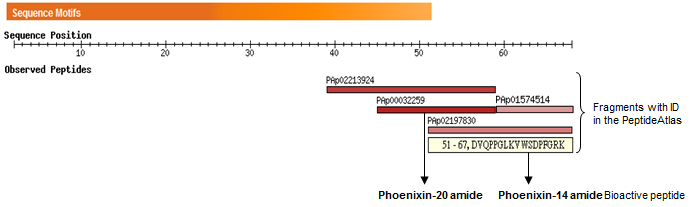
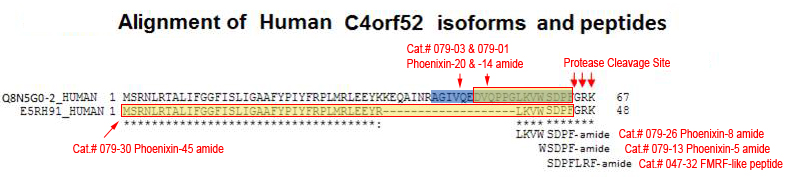
Alignment of amino acid sequences of homologous proteins from different species show high evolution sequence conservation. Based on potential proteolytic cleavage sites, three C-terminal hypothetical peptides are predicted: Phoenixin-20 amide, Phoenixin15 (Phoenixin-14-Gly), and Phoenixin-14 amide.
Abstract: The phoenixin (PNX) peptide is linked to the control of reproduction, food intake, stress, and inflammation. However, little is known about what regulates its gene and protein expression, information that is critical to understand the physiological role of PNX. In this review, we sum- marize what is known about the transcriptional control of Pnx and its receptor Gpr173. A main function of PNX is as a positive regulator of the hypothalamic-pituitary-gonadal axis, but there is a lack of research on its control by reproductive hormones and peptides. PNX is also associated with food intake, and its expression is linked to feeding status, fatty acids, and glucose. It is influenced by environmental and hormonal-induced stress. The regulation of Pnx in most contexts remains an enigma, in part due to conflicting and negative results. An extensive analysis of the response of the Pnx gene to factors related to reproduction, metabolism, stress, and inflammation is required. Analysis of the Pnx promoter and epigenetic regulation must be considered to understand how this level of control contributes to its pleiotropic effects. PNX is now linked to a broad range of functions, but more research on its gene regulation is required to understand its place in overall physiology and therapeutic potential.
McIlwraith EK, Zhang N, Belsham DD. The regulation of phoenixin: a fascinating multidimensional peptide. Journal of the Endocrine Society. 2022;6(2):bvab192.
Phoenixin is a pleiotropic peptide involved in reproduction, anxiety and recently also implicated in the control of food intake. Besides the 20-amino acid phoenixin, the 14-amino acid phoenixin-14 also shows bioactive properties. However, the expression sites of phoenixin-14 in the brain and peripheral tissues are not yet described in detail. Therefore, a mapping of the brain and peripheral tissues from male and female Sprague-Dawley rats with a specific phoenixin-14 antibody was performed using western blot and immunohistochemistry. High density of phoenixin-14 immunoreactivity was detected in the medial division of the brain central amygdaloid nucleus, in the spinal trigeminal tract and in the spinocerebellar tract as well as in cells between the crypts of duodenum, jejunum and ileum. Medium density immunoreactivity was observed in the bed nucleus of the stria terminalis, in the area postrema, the nucleus of the solitary tract and the dorsal motor nucleus of the vagus nerve as well as in the peripheral parts of the islets of Langerhans in the pancreas. A low density of phoenixin-14 immunoreactivity was detected in the arcuate nucleus, the supraoptic nucleus and the raphe pallidus. After pre-absorption of the antibody with phoenixin-14 peptide, no immunosignals were observed indicating specificity of the antibody. Taken together, the widespread distribution of phoenixin-14 immunoreactivity gives additional rise to the pleiotropic functions of the peptide such as possible effects in gastrointestinal motility, immune functions and glucose homeostasis.
Prinz P, Scharner S, Friedrich T, et al. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem Biophys Res Commun. 2017;493(1):195-201.
BACKGROUND: Alteration in energy expenditure or metabolism is the most accused risk issue for the onset and for the course of neurodegenerative cognitive disorders. Neuropeptides are suggested to be related with learning and memory. Phoenixin (PNX) is the most recently reported neuropeptide and we aimed to compare the plasma level in people with subjective memory complaints, patients with mild cognitive impairment, and mild Alzheimer's disease (AD).METHODS: Ninety two participants enrolled in the study. After screening tests, all participants were assessed with a neuropsychological battery for further cognitive evaluations. We used ELISA kit to assay the level of Human PNX.RESULTS: Patients with AD were significantly older than people in subjective memory complaint group (p = 0.02). There was no significant difference between groups according to gender (p = 0.435). Mean plasma PNX level was not significantly different between groups (p = 0.279). Mean plasma PNX level in MCI group was positively correlated with BMI (r = 0.402 and p = 0.028), serum HDL level (r = 0.454 and p = 0.012), blood systolic pressure (r = 0.428 and p = 0.018) and negatively correlated with logical memory (r=-0.335 and p=0.031). The mean plasma PNX level was positively correlated with immediate recall in subjective memory complaint group (r = 0.417 and p = 0.034).CONCLUSION: This study is the first studying the association of plasma PNX level and cognitive complaints or decline. The knowledge about the role, interaction, and physiological functions of PNX is lacking. Lower plasma PNX level might be important in prodromal stages as MCI and the predictive role of PNX should be investigated in further studies.
Yuruyen M, Gultekin G, Batun GC, et al. Int Psychogeriatr. 2017;:1-8.
Phoenixin (PNX) is a recently discovered neuropeptide shown to be involved in regulating the reproductive system, anxiety-related behaviors and pain though its receptor is still unknown. PNX-14, one of the endogenous active isoforms, is reported to regulate gonadotropin releasing hormone (GnRH) receptor expression and GnRH secretion. Because GnRH system is thought to be involved in the regulation of learning and memory processes, we hypothesized that PNX-14 might be mediate learning and memory. Here, we investigated the effects of PNX-14 in memory processes, using novel object recognition (NOR) and object location recognition (OLR) tasks. Our results revealed that intracerebroventricular (i.c.v.) injection of PNX-14 (25nmol) immediately after training not only facilitated memory formation, but also prolonged memory retention in both tasks. The memory-enhancing effects of PNX-14 were also seen when it was infused into the hippocampus. Moreover, these memory-improving effects of PNX-14 could be blocked by a GnRH receptor antagonist (Cetrorelix). The memory-improving effects of PNX-14 were not related to any effects on locomotor activity. Additionally, the results suggested that i.c.v. injection of PNX-14 mitigate the memory impairment induced by the amyloid-β1-42 (Aβ1-42) peptide and scopolamine. The present results indicate that PNX-14 facilitates memory formation and prolongs memory retention through activation of the GnRH receptor, and mitigates the memory-impairing effects of Aβ1-42 and scopolamine, suggesting that PNX-14 may be effective as a drug for enhancing memory and treating Alzheimer?s disease.
Jiang JH, He Z, Peng YL, et al. Brain Res. 2015;1629:298-308.
The process of ischemia/reperfusion (IR) in ischemic stroke often leads to significant cell death and permanent neuronal damage. Safe and effective treatments are urgently needed to mitigate the damage caused by IR injury. The naturally occurring pleiotropic peptide phoenixin 14 (PNX-14) has recently come to light as a potential treatment for IR injury. In the present study, we examined the effects of PNX-14 on several key processes involved in ischemic injury, such as pro-inflammatory cytokine expression, oxidative stress, and the related cascade mediated through the toll-like receptor 4 (TLR4) pathway, using BV2 microglia exposed to oxygen-glucose deprivation and reoxygenation (OGD/R). Our results demonstrate an acute ability of PNX-14 to regulate the expression levels of proinflammatory cytokines including tumor necrosis factor-? (TNF-?), interleukin-1? (IL-1?), and interleukin-6 (IL-6). PNX-14 also prevented oxidative stress by reducing the generation of reactive oxygen species (ROS) and increasing the level of the antioxidant glutathione (GSH). Importantly, PNX-14 inhibited high-mobility group box 1 (HMGB1)/TLR4/myeloid differentiation primary response 88 (MyD88)/nuclear factor-?B (NF-?B) signaling pathway, by inhibiting the activation of TLR4 and preventing the nuclear translocation of p65 protein. We further confirmed the cerebroprotective effects of PNX-14 in an MCAO rat model, which resulted in reduced infarct volume and decreased microglia activation. Together, the results of this study implicate a possible protective role of PNX-14 against various aspects of IR injury in vitro
Due to the dynamic development of molecular neurobiology and bioinformatic methods several novel brain neuropeptides have been identified and characterized in recent years. Contemporary techniques of selective molecular detection e.g. in situ Real-Time PCR, microdiffusion and some bioinformatics strategies that base on searching for single structural features common to diverse neuropeptides such as hidden Markov model (HMM) have been successfully introduced. A convincing majority of neuropeptides have unique properties as well as a broad spectrum of physiological activity in numerous neuronal pathways including the hypothalamus and limbic system. The newly discovered but uncharacterized regulatory factors nesfatin-1, phoenixin, spexin and kisspeptin have the potential to be unique modulators of stress responses and eating behaviour. Accumulating basic studies revelaed an intriguing role of these neuropeptides in the brain pathways involved in the pathogenesis of anxiety behaviour. Nesfatin-1, phoenixin, spexin and kisspeptin may also distinctly affect the energy homeostasis and modulate food intake not only at the level of hypothalamic centres. Moreover, in patients suffered from anxiety and anorexia nervosa a significant, sex-related changes in the plasma neuropeptide levels occurred. It should be therefore taken into account that the targeted pharmacomodulation of central peptidergic signaling may be potentially helpful in the future treatment of certain neuropsychiatric and metabolic disorders. This article reviews recent evidence dealing with the hypothetical role of these new factors in the anxiety-related circuits and pathophysiology of anorexia nervosa.
Pa?asz A, Janas-kozik M, Borrow A, Arias-carrión O, Worthington JJ. The potential role of the novel hypothalamic neuropeptides nesfatin-1, phoenixin, spexin and kisspeptin in the pathogenesis of anxiety and anorexia nervosa. Neurochem Int. 2018;113:120-136.
Phoenixin is an amidated neuropeptide, which is widely distributed in brain and periphery regions and is known for its key role in reproduction. Phoenixin-14 (PNX-14), one of the endogenous active isoforms, was reported to regulate pituitary gonadotrophin secretion by increasing the expression of the GnRH receptor mRNA. Studies showed that GnRH could regulate brain responses to anxiety. However, the role of PNX-14 in anxiety was largely unclear. Here, we investigated that the effects of PNX-14 in anxiety-related behavior in adult mice via the open field and elevated plus maze. PNX-14 was administered intracerebroventricularly (i.c.v.) in different doses (5, 10, 25 and 50nmol), and dose-dependently induced anxiolytic effects. Then this anxiolytic action was presented after PNX-14 injected into the anterior hypothalamic area (AHA), while PNX-14 infused into the amygdala did not exert anxiolytic effects. GnRH receptor antagonist (Cetrorelix) could significantly antagonize the anxiolytic effects of PNX-14, while Atosiban, a competitive vasopressin/oxytocin receptor antagonist could not. Moreover, PNX-14 could significantly lower the core temperature and Cetrorelix could block this effect of PNX-14. Additionally, the AHA infusion of PNX-14 (5nmol) increased the expression level of the GnRH mRNA in the hypothalamus and plasma concentrations of GnRH. Similarly, i.c.v. injection of PNX-20 also reduced the core temperature and exerted anxiolytic effects. Taken together, centrally injected PNX-14 generates anxiolytic effects in mice, via the activation of the AHA GnRH system.
Jiang JH, He Z, Peng YL, et al. Effects of Phoenixin-14 on anxiolytic-like behavior in mice. Behav Brain Res. 2015;286:39-48.
Abstract: Phoenixin-14 (PNX-14) is a newly identified neuropeptide with potential anti-inflammatory effects in the gastrointestinal tract. In this study, we evaluated the protective effect of PNX-14 against the formation of experimental indomethacin (IND)-induced duodenal ulcer. Thirty-two male Sprague-Dawley rats were randomly assigned to the four following study groups: (1) negative control (2) IND (7.5 mg/kg subcutaneous IND), (3) famotidine (FA) (7.5 mg/kg subcutaneous IND followed by 40 mg/kg intraperitoneal FA), and (4) PNX-14 (7.5 mg/kg subcutaneous IND followed by 50 µ/kg intraperitoneal PNX-14). Outcome measures included macroscopic evaluation of duodenal lesion, serum levels of IL-1ß, TNF-α, IL-6, and IL-12, and tissue biochemical parameters of oxidative stress, including malondialdehyde (MDA), myeloperoxidase (MPO) activity, superoxide dismutase (SOD) activity, and catalase activity. Results The macroscopic grade of duodenal lesions were significantly smaller in the PNX-14 group than in the IND group (p < 0.001). Serum inflammatory cytokines were significantly increased in the IND group. PNX-14 treatment significantly decreased the serum levels of inflammatory cytokines (p < 0.0001). Oxidative contents (MDA and MPO activity) were significantly smaller in the PNX-14 group compared with the IND group (p < 0.0001), while anti-oxidative contents (SOD and catalase activity) were significantly more (p < 0.0001). PNX-14 was superior to FA in several anti-inflammatory properties, such as inhibiting the release of inflammatory cytokines and increasing the catalase activity. PNX-14 showed significant protective effects against the formation of IND-induced duodenal ulcers. These results suggest a promising therapeutic implication for PNX-14 in the treatment of gastrointestinal inflammatory disorders.
Zandeh-Rahimi Y, Panahi N, Hesaraki S, Shirazi-Beheshtiha SH. Protective effects of phoenixin-14 peptide in the indomethacin-induced duodenal ulcer: an experimental study. Int J Pept Res Ther. 2022;28(1):43.
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases. The development of NAFLD is closely associated with hepatic lipotoxicity, inflammation, and oxidative stress. The new concept of NAFLD treatment is to seek molecular control of lipid metabolism and hepatic redox hemostasis. Phoenixin is a newly identified neuropeptide with pleiotropic effects. This study investigated the effects of phoenixin 14 against high-fat diet (HFD)-induced NAFLD in mice.
Yang F, Huang P, Shi L, Liu F, Tang A, Xu S. Phoenixin 14 Inhibits High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Experimental Mice. Drug Des Devel Ther. 2020;14:3865-3874
Abstract: There are examples of physiological conditions under which thirst is inappropriately exaggerated, and the mechanisms for these paradoxical ingestive behaviors remain unknown. We are interested in thirst mechanisms across the female life cycle and have identified a novel mechanism through which ingestive behavior may be activated. We discovered a previously unrecognized endogenous hypothalamic peptide, phoenixin (PNX), identified physiologically relevant actions of the peptide in brain and pituitary gland to control reproductive hormone secretion in female rodents, and in the process identified the previously orphaned G protein-coupled receptor Gpr173 to be a potential receptor for the peptide. Labeled PNX binding distribution in brain parallels areas known to be important in ingestive behaviors as well in areas where gonadal steroids feedback to control estrous cyclicity (Stein LM, Tullock CW, Mathews SK, Garcia-Galiano D, Elias CF, Samson WK, Yosten GLC, Am J Physiol Regul Integr Comp Physiol 311: R489–R496, 2016). We have demonstrated upregulation of Gpr173 during puberty, fluctuations across the estrous cycle, and, importantly, upregulation during the last third of gestation. It is during this hypervolemic, hyponatremic state that both vasopressin secretion and thirst are inappropriately elevated in humans. Here, we show that central administration of PNX stimulated water drinking in both males and females under ad libitum conditions, increased water drinking after overnight fluid deprivation, and increased both water and 1.5% NaCl ingestion under fed and hydrated conditions. Importantly, losartan pretreatment blocked the effect of PNX on water drinking, and knockdown of Gpr173 by use of short interfering RNA constructs significantly attenuated water drinking in response to overnight fluid deprivation. These actions, together with the stimulatory action of PNX on vasopressin secretion, suggest that this recently discovered neuropeptide may impact the recruitment of critically important neural circuits through which ingestive behaviors and endocrine mechanisms that maintain fluid and electrolyte homeostasis are regulated.
Haddock CJ, Almeida-Pereira G, Stein LM, Yosten GLC, Samson WK. A novel regulator of thirst behavior: phoenixin. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2020;318(6):R1027-R1035.
Phoenixin (PNX) neuropeptide is a cleaved product of the Smim20 protein. Its most common isoforms are the 14- and 20-amino acid peptides. The biological functions of PNX are mediated via the activation of the GPR173 receptor. PNX plays an important role in the central nervous system (CNS) and in the female reproductive system where it potentiates LH secretion and controls the estrus cycle. Moreover, it stimulates oocyte maturation and increases the number of ovulated oocytes. Nevertheless, PNX not only regulates the reproduction system but also exerts anxiolytic, anti-inflammatory, and cell-protective effects. Furthermore, it is involved in behavior, food intake, sensory perception, memory, and energy metabolism. Outside the CNS, PNX exerts its effects on the heart, ovaries, adipose tissue, and pancreatic islets. This review presents all the currently available studies demonstrating the pleiotropic effects of PNX.
Billert M, Rak A, Nowak KW, Skrzypski M. Phoenixin: more than reproductive peptide. IJMS. 2020;21(21):8378.
Osteogenic differentiation is critical to bone homeostasis, and its imbalance plays a key role in the progression of osteoporosis. Osteoblast cells are responsible for synthesizing new bone tissue, and understanding how to control osteoblastic differentiation is vital to the treatment of osteoporosis. Herein, we show that GPR173 signaling is involved in the regulation of osteoblastic differentiation in MC3T3-E1 cells. Our data reveals that GPR173 is abundantly expressed in MC3T3-E1 cells, and its expression is inducible upon the introduction of osteogenic media. The activation of GPR173 by its selective agonist phoenixin 20 induces the expression of several osteoblast signature genes including collagen type 1 alpha 1 (Col-I), osteocalcin (OCN), alkaline phosphatase (ALP) as well as increased matrix mineralization and ALP activity, suggesting that the activation of GPR173 promotes osteoblastic differentiation. Moreover, we show that the effect of phoenixin 20 is mediated by its induction on the key regulator runt-Related Transcription Factor 2 (Runx2). Mechanistically, we display that the action of phoenixin 20 requires the activation of MAPK kinase p38, and deactivation of p38 by its inhibitor SB203580 weakens the phoenixin 20-mediated induction of RUNX-2, ALP, and matrix mineralization. Silencing of GPR173 attenuates phoenixin 20-mediated osteoblastic differentiation, indicating its dependence on the receptor. Collectively, our study reveals a new role of GPR173 and its agonist phoenixin 20 in osteoblastic differentiation.
Gu Z, Xie D, Ding R, Huang C, Qiu Y. GPR173 agonist phoenixin 20 promotes osteoblastic differentiation of MC3T3-E1 cells. Aging. 2021;13(4):4976-4985.
Phoenixin, a recently discovered 20-amino acid peptide was implicated in reproduction. However, the expression in food intake-regulatory nuclei such as the paraventricular nucleus, the arcuate nucleus and the nucleus of the solitary tract suggests an implication of phoenixin in food intake regulation. Therefore, we investigated the effects of phoenixin-14, the shorter form of phoenixin, on food intake following intracerebroventricular (icv) and intraperitoneal (ip) injection in ad libitum fed male Sprague-Dawley rats. Phoenixin-14 injected icv (0.2, 1.7 or 15nmol/rat) during the light phase induced a dose-dependent increase of light phase food intake reaching significance at a minimum dose of 1.7 nmol/rat (+72%, p<0.05 vs. vehicle) used for all further analyses. Assessment of the food intake microstructure showed an icv phoenixin-14-induced increase in meal size (+51%), meal duration (+157%), time spent in meals (+182%) and eating rate (+123%), while inter-meal intervals (-42%) and the satiety ratio (-64%) were decreased compared to vehicle (p<0.05). When injected icv during the dark phase, no modulation of food intake was observed (p>0.05). The light phase icv phoenixin-14-induced increase of water intake did not reach statistical significance compared to vehicle (+136%, p>0.05). The increase of food intake following icv phoenixin-14 was not associated with a significant alteration of grooming behavior (0.4-fold, p=0.377) or locomotion (6-fold, p=0.066) compared to vehicle. When injected ip at higher doses (0.6, 5nmol/kg or 45nmol/kg body weight) during the light phase, phoenixin-14 did not affect food intake (p>0.05). In summary, phoenixin-14 exerts a centrally-mediated orexigenic effect.
Schalla M, Prinz P, Friedrich T, et al. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides. 2017;96:53-60.
Sexual maturation and maintenance of reproductive function are regulated by neurohormonal communication between the hypothalamus, pituitary, and gonads (referred to as the HPG axis). Phoenixin (PNX) is a newly identified, endogenous peptide abundantly produced in the hypothalamus and shown to be an important mediator of ovarian cyclicity. However, the underlying mechanisms by which phoenixin functions within the HPG axis are unknown. Previous in vitro studies demonstrated a direct action of PNX on gonadotrophs to potentiate gonadotrophin-releasing hormone (GnRH) induced luteinizing hormone (LH) secretion. Therefore, we hypothesized that centrally derived phoenixin regulates the preovulatory LH surge required for ovarian cyclicity. We observed a significant dose-related increase in the level of plasma LH in diestrous, female rats that were given an intracerebroventricular injection of PNX compared with vehicle-treated controls. While this suggests that even under low-estrogen conditions, PNX acts centrally to stimulate the HPG axis, further characterization is contingent on the elucidation of its cognate receptor. Using the "deductive ligand receptor matching strategy," we identified the orphan G protein-coupled receptor, Gpr173, as our top candidate. In cultured pituitary cells, siRNA-targeted compromise of Gpr173 abrogated PNX's action to potentiate GnRH-stimulated LH secretion. In addition, siRNA-mediated knockdown of endogenous Gpr173, which localized to several hypothalamic sites related to reproductive function, not only significantly extended the estrous cycle but also prevented the PNX-induced LH secretion in diestrous, female rats. These studies are the first to demonstrate a functional relationship between PNX and Gpr173 in reproductive physiology and identify a potential therapeutic target for ovulatory dysfunction.
Stein LM, Tullock CW, Mathews SK, et al. Am J Physiol Regul Integr Comp Physiol. 2016;311(3):R489-96.
Introduction: Dynamic development of the biotechnology results in discovery and description of new neuropeptides, localized in different areas of the brain, which have a brought, multidirectional spectrum of activities, performed at the level of different neuronal pathways. Aim: (1) Review of literature concerning with newly-discovered neuropeptide – phoenixin (PNX) and (2) assessment of its distribution in hypothalamic structures of adult rats. Material and methods: (1) A search of available databases for articles about PNX; (2) evaluation of the distribution of PNX in hypothalamic structures of adult Sprague-Dawley (SD) rats with immunohistochemistry (IHC) and immunofluorescence (IFC), using the original antibody from Phoenix Pharmaceuticals. Results: (1) We found only 2 original papers and one proceeding; (2) we confirmed the presence of PNX in various structures of the hypothalamus of SD rats, both by IHC and IFC. Conclusions: PNX is a newly-discovered and still extremely poorly known neuropeptide, representing a unique class of hypothalamic regulatory factors. So far, we know only that it regulates the secretion of pituitary gonadotropins by modulating the expression of the receptor for gonadotropin-releasing hormone (GnRH-R). An initial study suggests, that PNX sensitize the pituitary to the action of releasing factors, rather than directly stimulates the exocytosis of secretory vesicles to pituitary endocrine cells. Immunohistochemical studies revealed PNX immunoreactivity in the rat hypothalamus, superficial dorsal horn, spinal trigeminal tract, nucleus of the solitary tract; and in a population of dorsal root, trigeminal and nodose ganglion cells. It was also observed that exogenously administered PNX may preferentially suppress visceral as opposed to thermal pain. Recent reports suggest that the mechanism of signal transduction activated by PNX is MAPK/ERK pathway.
Conference: Conference: IV Zjazd Polskiego Towarzystwa Neuroendokrynologii, At ?ód?, Volume: Endokrynologia Polska, 2014; 65(5): 430-431Conference: Conference: IV Zjazd Polskiego Towarzystwa Neuroendokrynologii, At ?ód?, Volume: Endokrynologia Polska, 2014; 65(5): 430-431
The hypothalamus regulates a number of autonomic functions essential for homeostasis; therefore, investigations concerning hypothalamic neuropeptides and their functions and distribution are of great importance in contemporary neuroscience. Recently, novel regulatory factors expressed in the hypothalamus have been discovered, of which nesfatin-1 and phoenixin (PNX), show intriguing similarities in their brain distributions. There are currently few studies characterizing PNX expression, so it is imperative to accurately trace its localization, with particular attention to the hypothalamic nuclei and nesfatin-1 co-expression. Using fluorescence and classical immunohistochemical stainings on adult rat brain, we visualized the potential co-expression of nesfatin-1 and PNX immunoreactive cells. We have demonstrated a distinct PNX-immunoreactivity in 21-32% of cells in the arcuate nucleus, paraventricular nucleus, ventromedial and lateral hypothalamus. Nesfatin-1 expression reached 45-68% of all neurons in the same sites, while co-expression was strikingly seen in the vast majority (70-86%) of PNX-immunoreactive neurons in the rat hypothalamic nuclei. Our results demonstrate for the first time, a wide distribution of PNX in the hypothalamus which could implicate a potential functional relationship with nesfatin-1, possibly in the regulation of the hypothalamic-pituitary-gonadal axis or other autonomic functions, which require further study.
Pa?asz A, Rojczyk E, Bogus K, Worthington JJ, Wiaderkiewicz R. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical study. Neurosci Lett. 2015;592:17-21.
Normal anterior pituitary function is essential for fertility. Release from the gland of the reproductive hormones luteinising hormone and follicle-stimulating hormone is regulated primarily by hypothalamically-derived gonadotrophin-releasing hormone (GnRH), although other releasing factors (RF) have been postulated to exist. Using a bioinformatic approach, we have identified a novel peptide, phoenixin, that regulates pituitary gonadotrophin secretion by modulating the expression of the GnRH receptor, an action with physiologically relevant consequences. Compromise of phoenixin in vivo using small interfering RNA resulted in the delayed appearance of oestrus and a reduction in GnRH receptor expression in the pituitary. Phoenixin may represent a new class of hypothalamically-derived pituitary priming factors that sensitise the pituitary to the action of other RFs, rather than directly stimulating the fusion of secretary vesicles to pituitary membranes.
Yosten GL, Lyu RM, Hsueh AJ, et al. A novel reproductive peptide, phoenixin. J Neuroendocrinol. 2013;25(2):206-15.
BACKGROUND: Alteration in energy expenditure or metabolism is the most accused risk issue for the onset and for the course of neurodegenerative cognitive disorders. Neuropeptides are suggested to be related with learning and memory. Phoenixin (PNX) is the most recently reported neuropeptide and we aimed to compare the plasma level in people with subjective memory complaints, patients with mild cognitive impairment, and mild Alzheimer's disease (AD). METHODS: Ninety two participants enrolled in the study. After screening tests, all participants were assessed with a neuropsychological battery for further cognitive evaluations. We used ELISA kit to assay the level of Human PNX. RESULTS: Patients with AD were significantly older than people in subjective memory complaint group (p = 0.02). There was no significant difference between groups according to gender (p = 0.435). Mean plasma PNX level was not significantly different between groups (p = 0.279). Mean plasma PNX level in MCI group was positively correlated with BMI (r = 0.402 and p = 0.028), serum HDL level (r = 0.454 and p = 0.012), blood systolic pressure (r = 0.428 and p = 0.018) and negatively correlated with logical memory (r=-0.335 and p=0.031). The mean plasma PNX level was positively correlated with immediate recall in subjective memory complaint group (r = 0.417 and p = 0.034). CONCLUSION: This study is the first studying the association of plasma PNX level and cognitive complaints or decline. The knowledge about the role, interaction, and physiological functions of PNX is lacking. Lower plasma PNX level might be important in prodromal stages as MCI and the predictive role of PNX should be investigated in further studies.
Yuruyen M, Gultekin G, Batun GC, et al. Does plasma phoenixin level associate with cognition? Comparison between subjective memory complaint, mild cognitive impairment, and mild Alzheimer’s disease. Int Psychogeriatr.
Phoenixin is a pleiotropic peptide involved in reproduction, anxiety and recently also implicated in the control of food intake. Besides the 20-amino acid phoenixin, the 14-amino acid phoenixin-14 also shows bioactive properties. However, the expression sites of phoenixin-14 in the brain and peripheral tissues are not yet described in detail. Therefore, a mapping of the brain and peripheral tissues from male and female Sprague-Dawley rats with a specific phoenixin-14 antibody was performed using western blot and immunohistochemistry. High density of phoenixin-14 immunoreactivity was detected in the medial division of the brain central amygdaloid nucleus, in the spinal trigeminal tract and in the spinocerebellar tract as well as in cells between the crypts of duodenum, jejunum and ileum. Medium density immunoreactivity was observed in the bed nucleus of the stria terminalis, in the area postrema, the nucleus of the solitary tract and the dorsal motor nucleus of the vagus nerve as well as in the peripheral parts of the islets of Langerhans in the pancreas. A low density of phoenixin-14 immunoreactivity was detected in the arcuate nucleus, the supraoptic nucleus and the raphe pallidus. After pre-absorption of the antibody with phoenixin-14 peptide, no immunosignals were observed indicating specificity of the antibody. Taken together, the widespread distribution of phoenixin-14 immunoreactivity gives additional rise to the pleiotropic functions of the peptide such as possible effects in gastrointestinal motility, immune functions and glucose homeostasis.
Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochemical and Biophysical Research Communications. 2017;493(1):195-201.
Phoenixin (PNX) is a recently discovered neuropeptide shown to be involved in regulating the reproductive system, anxiety-related behaviors and pain though its receptor is still unknown. PNX-14, one of the endogenous active isoforms, is reported to regulate gonadotropin releasing hormone (GnRH) receptor expression and GnRH secretion. Because GnRH system is thought to be involved in the regulation of learning and memory processes, we hypothesized that PNX-14 might be mediate learning and memory. Here, we investigated the effects of PNX-14 in memory processes, using novel object recognition (NOR) and object location recognition (OLR) tasks. Our results revealed that intracerebroventricular (i.c.v.) injection of PNX-14 (25nmol) immediately after training not only facilitated memory formation, but also prolonged memory retention in both tasks. The memory-enhancing effects of PNX-14 were also seen when it was infused into the hippocampus. Moreover, these memory-improving effects of PNX-14 could be blocked by a GnRH receptor antagonist (Cetrorelix). The memory-improving effects of PNX-14 were not related to any effects on locomotor activity. Additionally, the results suggested that i.c.v. injection of PNX-14 mitigate the memory impairment induced by the amyloid-?1-42 (Aβ1-42) peptide and scopolamine. The present results indicate that PNX-14 facilitates memory formation and prolongs memory retention through activation of the GnRH receptor, and mitigates the memory-impairing effects of A?1-42 and scopolamine, suggesting that PNX-14 may be effective as a drug for enhancing memory and treating Alzheimer's disease.
Jiang JH, He Z, Peng YL, et al. Phoenixin-14 enhances memory and mitigates memory impairment induced by Aβ1-42 and scopolamine in mice. Brain Res. 2015;1629:298-308.
Abstract: The most common uterine diseases affecting bitches are cystic endometrial hyperplasia (CEH) and pyometra. The neuropeptide phoenixin (PNX) and its receptor (GPR173) are potential key factors involved in the proliferative and inflammatory regulation of the reproductive system in females. This study aimed to evaluate the expression of PNX and GPR173 by qPCR, western blot and immunofluorescence assays in the endometrium of bitches suffering from CEH or pyometra compared to clinically healthy females. Additionally, PNX and progesterone (P4) plasma concentrations were analysed. The results showed a significantly lower expression levels of PNX and GPR173 (mRNA and protein production) in bitches with the CEH or pyometra groups compared to healthy animals. Immunofluorescence staining examination also confirmed a lower concentration of PNX and GPR173 signals in bitches with pathological uteri. Moreover, a lower concentration of PNX blood levels in bitches suffering from pyometra was observed. The PNX concentration was negatively correlated with P4 but only in healthy bitches. These results illustrate that the development of canine uterine disorders may cause a lower expression of PNX and its receptor GPR173.
Rybska M, Billert M, Skrzypski M, et al. Canine cystic endometrial hyperplasia and pyometra may downregulate neuropeptide phoenixin and GPR173 receptor expression. Animal Reproduction Science. 2022;238:106931.
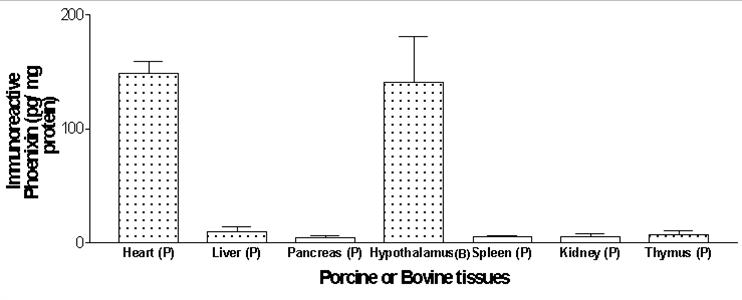
The level of immunoreactive Phoenixin in porcine (P) or bovine (B) tissues were measured by using a specific RIA kit that recognizes both Phoenixin-20 amide and Phoenixin-14 amide.
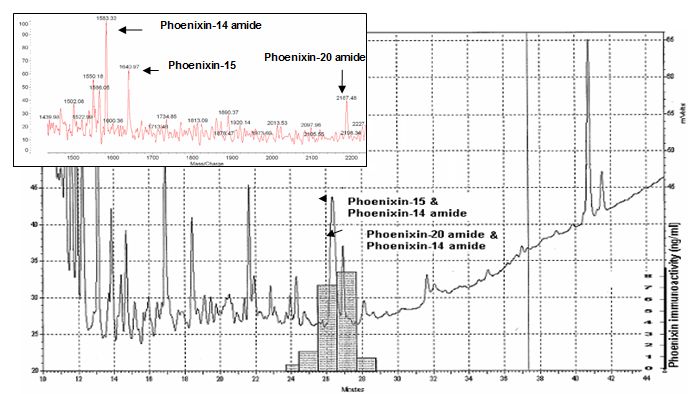
HPLC profile and immunoreactivity of isolated peptides- In the heart homogenate extracts, the major peaks of phoenixin immunoreactive fractions were from the elutes at 26 and 27 min of the 1st HPLC column. The major immunoreactive fraction at 27 min of 1st HPLC contains peptide ions at MW 1583, 1641 and 2187 which were corresponding to the theoretical molecular weight of the peptides, Phoenixin-14 amide, Phoenixin-15 and Phoenixin-20 amide. In addition, the synthetic peptides, Phoenixin-14 amide, Phoenixin 15 and Phoenixin-20 amide eluted from the HPLC at 27 min and showed the same peak positions around the MW of 1583, 1641 and 2184 in mass spectrometry.
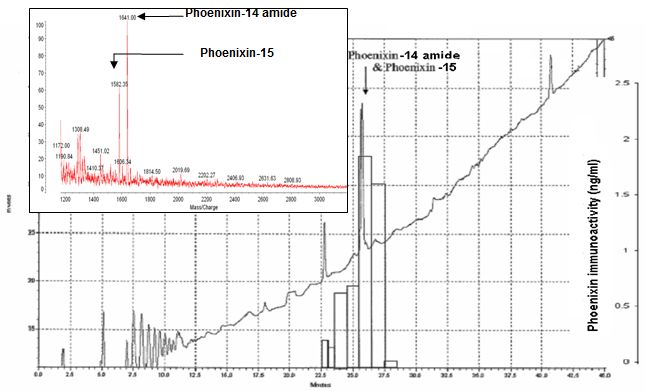
The 2nd HPLC profile, immunoreactivity and Mass spectrometry to identify the isolated peptides- The fractions with highest immunoreactivity in 2nd HPLC were from the peak between 26 min and 27 min. The fraction collected at 26 min, the isolated peptide identified by Mass spectrometer were at MW 1582.3 and 1641 which represent Phoenixin-14 amide and Phoenixin-15.
Social Network Confirmation