The N-terminal fragment of pro-opiomelancortin (POMC) has been shown previously to act as an adrenal mitogen. However, little is known about the molecular mechanisms by which mitogenesis is stimulated, although it has been shown that N-POMC(1-28) stimulates the ERK pathway in human H295R cells. We have investigated signaling stimulated by N-POMC(1-28) and N-POMC(1-49) in the mouse Y1 cell line and found that both peptides stimulate ERK phosphorylation with maximal stimulation being achieved within 5min. Similar results were observed for both MEK and c-Raf phosphorylation, although N-POMC(1-49) stimulated the phosphorylation of Akt more robustly than N-POMC(1-28). We also investigated the expression of tyrosine kinase receptors in adrenal cells. PCR utilizing degenerate primers was performed on cDNA from both Y1 cells and rat adrenal tissue. Sequencing of 114 clones from each cDNA population revealed the expression of a number of receptors, several of which have not been described previously in the adrenal.
Pepper DJ, Bicknell AB. The stimulation of mitogenic signaling pathways by N-POMC peptides. Mol Cell Endocrinol. 2009;300(1-2):77-82
Bioactive peptides derived from the prohormone, pro-opiomelanocortin (POMC), are generated in neurons of the hypothalamus and act as endogenous ligands for the melanocortin-4 receptor (MC4R), a key molecule underlying appetite control and energy homeostasis. It is therefore important to understand many aspects of POMC gene regulation in the brain, as pharmacological manipulation of POMC expression/processing could be a potential strategy to combat obesity. Most studies that have analysed POMC gene expression in the hypothalamus have focused on gene transcription experiments. Ultimately, however, factors that regulate post-translational processing and secretion of peptides will have most bearing on melanocortin signalling. This article focuses on (a) current evidence that POMC is involved in obesity, (b) how POMC transcription is regulated in the hypothalamus, (c) the mechanism by which proteolytic processing of POMC is controlled in the hypothalamus and what peptides are produced and (d) which POMC-derived peptides are the most potent ligands at the melanocortin receptor in vitro and in vivo. It seems that post-translational cleavage of POMC in the hypothalamus may be regulated with respect to energy requirement. We predict that further research into hypothalamic POMC processing, and the proteolytic enzymes involved, may yield important new clues on how flux through the MC4R pathway is regulated.
Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol. 2002;172(3):411-21
Significant progress in our understanding of the mechanisms of weight homeostasis has been made by studying the many genetic mouse models of obesity. Positional cloning in the obese mouse led to the discovery of leptin as a feedback messenger indicating the adequacy of peripheral energy stores. This was the first in a series of important advances in this field. Shortly after this discovery, two research laboratories presented evidence for the role of hypothalamic pro-opiomelanocortinergic (POMC) neurons as important mediators in the regulation of feeding behavior, insulin levels and, ultimately, body weight. One of these mouse obesity models, the lethal yellow mouse, constitutively overexpresses the agouti protein, an endogenous antagonist of both the melanocortin 1 (MC1) and melanocortin 4 (MC4) receptors. A second mouse obesity model was created by knocking out the MC4 receptor. Investigations using both the autosomal dominant lethal yellow mouse and MC4 receptor knockout mouse have provided clear evidence for the role of hypothalamic POMC neurons and the MC4 receptor in the regulation of weight homeostasis in the rodent. Furthermore, the recent discovery of agouti-related protein (AGRP), an agouti-like peptide naturally found in the hypothalamus, provides further evidence for the importance of POMC neurons in the regulation of weight. Although the significance of central POMC and AGRP in the rodent is apparent, the role of POMC neurons in the regulation of weight and feeding behavior in humans is only now being appreciated.
Boston BA. Pro-opiomelanocortin and weight regulation: from mice to men. J Pediatr Endocrinol Metab. 2001;14 Suppl 6:1409-16
The NH2-terminal region of pro-opiomelanocortin (POMC) is highly conserved across species, having two disulfide bridges that cause the formation of an amphipathic hairpin loop structure between the 2nd and 3rd cysteine residues (Cys8 to Cys20). The role that the NH2-terminal region of pro-opiomelanocortin plays in acting as a molecular sorting signal for the regulated secretory pathway was investigated by using site-directed mutagenesis either to disrupt one or more of the disulfide bridges or to delete the amphipathic loop entirely. When POMC was expressed in Neuro-2a cells, ACTH immunoreactive material was localized in punctate secretory granules in the cell body and along the neurites, with heavy labeling at the tips. ACTH was secreted from these POMC-transfected cells in a regulated manner. Disruption of both disulfide bridges or the second disulfide bridge or removal of the amphipathic hairpin loop resulted in constitutive secretion of the mutant POMC from the cells and a lack of punctate secretory granule immunostaining within the cells. We have modeled the NH2-terminal POMC Cys8 to Cys20 domain and have identified it as an amphipathic loop containing four highly conserved hydrophobic and acidic amino acid residues (Asp10-Leu11-Glu14-Leu1). Thus the sorting signal for POMC to the regulated secretory pathway appears to be encoded by a specific conformational motif comprised of a 13-amino acid amphipathic loop structure stabilized by a disulfide bridge, located at the NH2 terminus of the molecule.
Cool DR, Fenger M, Snell CR, Loh YP. Identification of the sorting signal motif within pro-opiomelanocortin for the regulated secretory pathway. J Biol Chem. 1995;270(15):8723-9
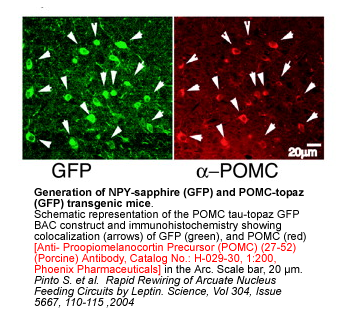
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| RK-029-30 | POMC (27-52) (Porcine) - RIA Kit | 125 tubes | $880 |
| FR-G-029-30 | POMC (27-52) (Porcine) - Rhodamine Labeled Purified IgG | 100 µl | $635 |
| WBK-029-30 | POMC (27-52) (Porcine) - Western Blot Kit | 1 kit | $777 |
Social Network Confirmation