P518, SP9155 (AQ27, GPR103), other RF amide peptides
QRFP 43 is a RFamide peptide present in the ventromedial nucleus (VMN) and lateral hypothalamus. It stimulates food intake in mice and its chronic infusion induces hyperphagia, reduced thermogenesis, and obesity. In this experiment, we measured it in the VMN and lateral hypothalamus of Long-Evans rats fed either a high-fat (HF), control, or low-fat (LF) diet in parallel with plasma leptin, adiposity, and energy intake. After 8weeks of ad libitum diet intake, energy intake of HF rats was similar to that of control rats. In the VMN, QRFP 43 was completely undetectable in HF rats and its tissue concentration in control rats was significantly lower than in LF rats (p<0.03). HF rats had higher levels of leptin than control rats (+24%; p<0.03) and than LF rats (+42%; p<0.002). The QRFP 43 concentration in the VMN was inversely correlated with plasma leptin (r=-0.34; P<0.04) and with the adipogenic index of the diet (p<0.02) but not with insulin. We conclude that the decrease of the orexigenic drive mediated by QRFP 43 could contribute to the normalization of caloric intake in HF diet fed rats. QRFP 43 might play a role downstream of leptin in the regulation of feeding behavior.
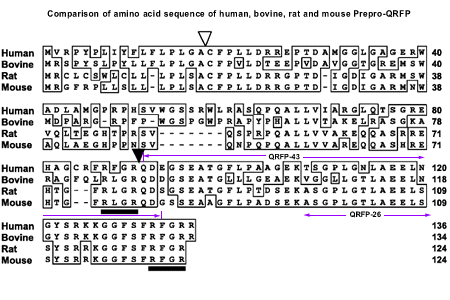
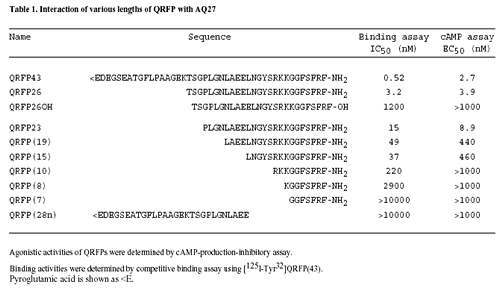
Pyrogultamylated arginine-phenylalanineamide peptide (QRFP) is strongly conserved across species and is a member of the family of RFamide-related peptides, with the motif Arg-Phe-NH(2) at the C-terminal end. The precursor peptide for QRFP generates a 26-amino acid peptide (QRFP-26) and a 43-amino acid peptide (QRFP-43), both of which bind to the G protein-coupled receptor, GPR103. Recently, QRFP has been characterized in rats, mice and humans and has been reported to have orexigenic properties. In rodents, prepro-QRFP mRNA is expressed in localized regions of the mediobasal hypothalamus, a region implicated in feeding behavior. Increased intake of a high fat diet contributes to increased weight gain and obesity. Therefore, the current experiments investigated the effects of QRFP administration in rats and the effects of a high fat diet on prepro-QRFP mRNA and GPR103 receptor mRNA levels. Intracerebroventricular administration of QRFP-26 (3.0nM, 5.0nM) and QRFP-43 (1.0nM, 3.0nM) dose-dependently increased 1h, 2h, and 4h cumulative intake of high fat (55% fat), but not low fat (10% fat) diet. In Experiment 2, hypothalamic prepro-QRFP mRNA levels and GPR103 receptor mRNA levels were measured in rats fed a high fat or a low fat diet for 21 days. Prepro-QRFP mRNA was significantly increased in the ventromedial nucleus/arcuate nucleus of the hypothalamus of rats fed a high fat diet compared to those fed a low fat diet, while GPR103 mRNA levels were unchanged. These findings suggest that QRFP is a regulator of dietary fat intake and is influenced by the intake of a high fat diet.
QRFP, an RF amide peptide, was recently identified as an endogenous ligand of an orphan G protein-coupled receptor, GPR103. Recent investigation revealed that acute intracerebroventricular (ICV) administration of QRFP26/P518/26RFa, a constitutive part of QRFP43 (43-amino acid-residue form of QRFP), increases appetite in mice, but its role in long-term energy homeostasis remains unknown. In the present study, we examined the effects of chronic administration of QRFP43 on feeding behavior, body weight regulation and energy expenditure in mice. ICV infusion of QRFP43 for 13 days resulted in a significant increase in body weight and fat mass with hyperphagia. Weight gain and hyperphagia were more evident when mice were fed a moderately high-fat diet. Pair-feeding of QRFP43-infused mice did not increase body weight, but significantly increased fat mass and plasma concentrations of insulin, leptin and cholesterol when compared with controls. Moreover, significant decreases in rectal temperature and expression of brown adipose tissue uncoupling protein-1 mRNA were observed in QRFP43-infused ad libitum- and pair-fed mice. The present results suggest that QRFP plays an important role in energy homeostasis by regulating appetite and energy expenditure.
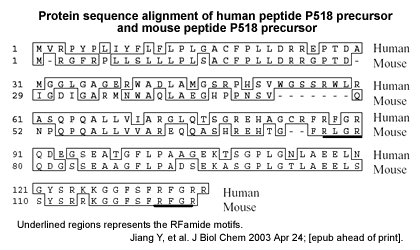
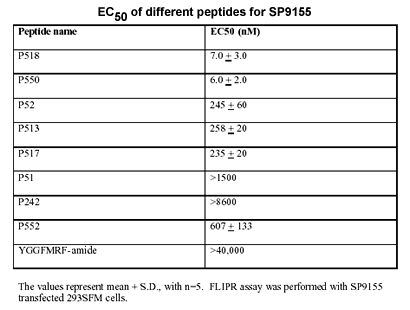
Orphan G-protein coupled receptors are a large class of receptors whose cognate ligands are unknown. SP9155 (also referred to as AQ27 and GPR103) is an orphan G-protein coupled receptor originally cloned from a human brain cDNA library. SP9155 was found to be predominantly expressed in brain, heart, kidney, retina and testis. Phylogenetic analysis shows that SP9155 shares high homology with Orexin, NPFF and CCK receptors, but identification of the endogenous ligand for SP9155 has not been reported. In this study, we have used a novel method to predict peptides from genome databases. From these predicted peptides, a novel RF-amide peptide, P52 was shown to selectively activate SP9155-transfected cells. We subsequently cloned the precursor gene of the P52 ligand and characterized the activity of other possible peptides encoded by the precursor. This revealed an extended peptide, P518, which exhibited high affinity for SP9155 (EC50 = 7 nM). mRNA expression analysis revealed that the peptide P518 precursor gene is predominantly expressed in various brain regions, coronary arteries, thyroid and parathyroid glands, large intestine, colon, bladder, testes and prostate. These results indicate the existence of a novel RF-amide neuroendocrine peptide system, and suggest that SP9155 is likely the relevant G-protein coupled receptor for this peptide.
SUMMARY: We searched for peptidic ligands for orphan G protein-coupled receptors utilizing a human genome database and identified a new gene encoding a preproprotein that could generate a peptide. This peptide consisted of 43 amino acid residues that starting from N-terminal pyroglutamic acid and ending at C-terminal arginine-phenylalanin-amide. We therefore named it QRFP after pyroglutamylated arginine-phenylalanine-amide peptide. We subsequently searched for its receptor and found that Chinese hamster ovary cells expressing an orphan G protein-coupled receptor, AQ27, specifically responded to QRFP. We analyzed tissue distributions of QRFP and its receptor mRNAs in rats utilizing quantitative reverse transcription-polymerase chain reaction and in situ hybridization. QRFP mRNA was highly expressed in the hypothalamus and its receptor mRNA in the adrenal gland. The intravenous administration of QRFP caused the release of aldosterone, suggesting that QRFP and its receptor have a regulatory function in the rat adrenal gland.
Related Products



Social Network Confirmation