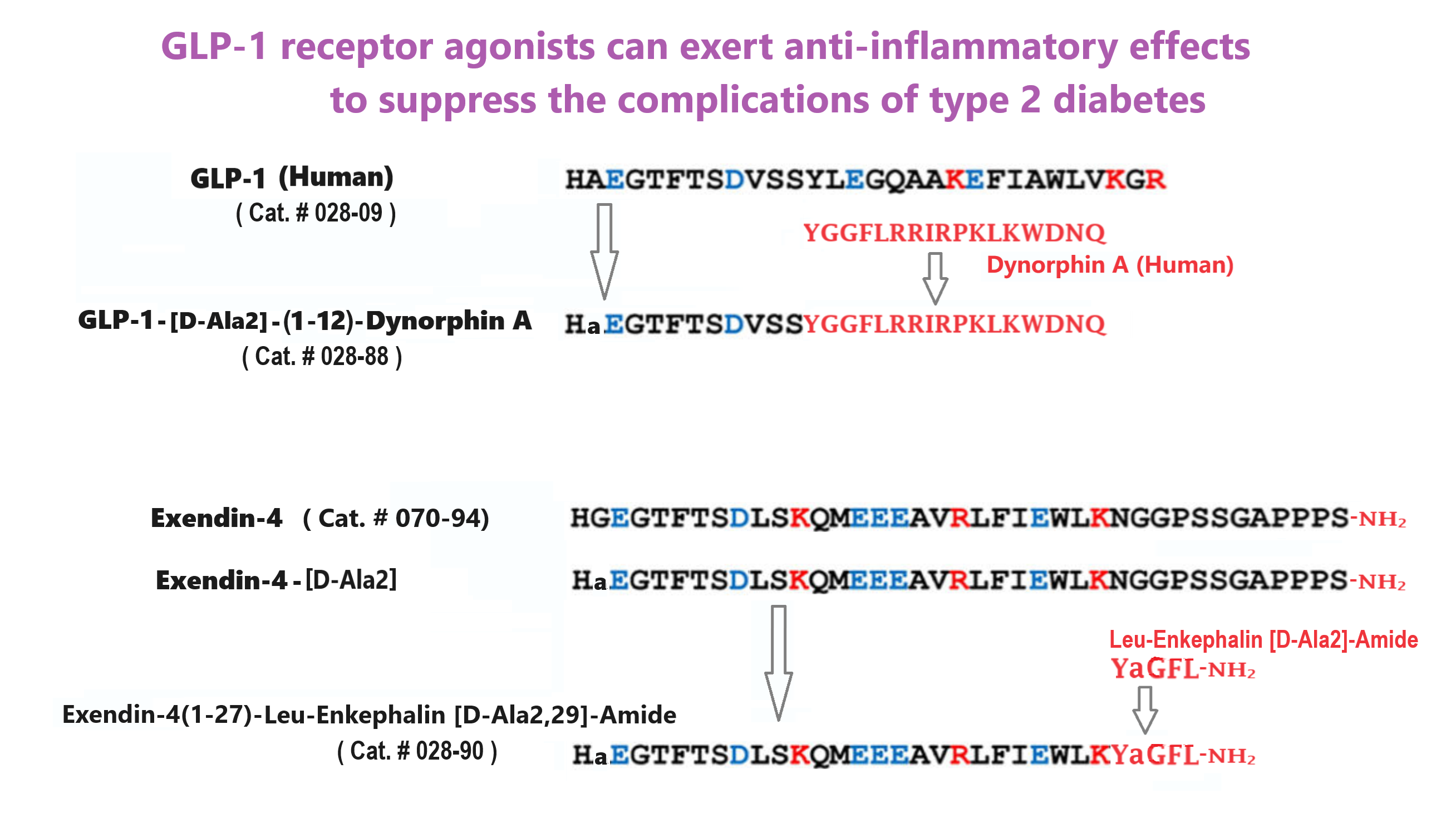
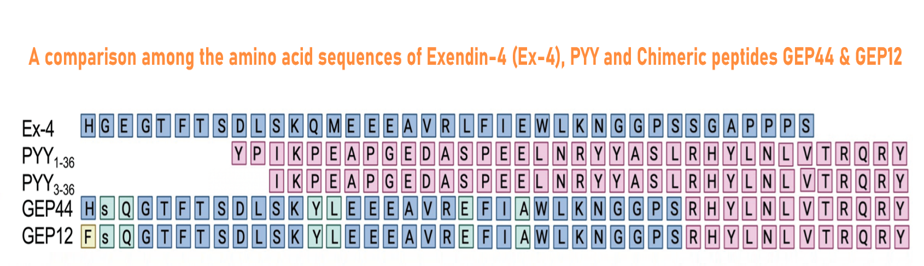
GEP44 (Cat. # 028-97), GEP12 (Cat. # 028-98) are made according the reference and Figure from:
Sci Rep. 2023 Jun 12;13(1):9554. doi: 10.1038/s41598-023-36178-1
Abstract: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) exert anti-inflammatory effects relevant to the chronic complications of type 2 diabetes. Although GLP-1RAs attenuate T cell-mediated gut and systemic inflammation directly through the gut intraepithelial lymphocyte GLP-1R, how GLP-1RAs inhibit systemic inflammation in the absence of widespread immune expression of the GLP-1R remains uncertain.
Wong CK, McLean BA, Baggio LL, et al. Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation. Cell Metabolism. 2024;36(1):130-143.e5.
Abstract: Mechanisms underlying long-term sustained weight loss and glycemic normalization after obesity surgery include changes in gut hormone levels, including glucagon-like peptide 1 (GLP-1) and peptide YY (PYY). We demonstrate that two peptide biased agonists (GEP44 and GEP12) of the GLP-1, neuropeptide Y1, and neuropeptide Y2 receptors (GLP-1R, Y1-R, and Y2-R, respectively) elicit Y1-R antagonist-controlled, GLP-1R-dependent stimulation of insulin secretion in both rat and human pancreatic islets, thus revealing the counteracting effects of Y1-R and GLP-1R agonism. These agonists also promote insulin-independent Y1-R-mediated glucose uptake in muscle tissue ex vivo and more profound reductions in food intake and body weight than liraglutide when administered to diet-induced obese rats. Our findings support a role for Y1-R signaling in glucoregulation and highlight the therapeutic potential of simultaneous receptor targeting to achieve long-term benefits for millions of patients.
Chichura KS, Elfers CT, Salameh TS, et al. A peptide triple agonist of GLP-1, neuropeptide Y1, and neuropeptide Y2 receptors promotes glycemic control and weight loss. Sci Rep. 2023;13(1):9554.
Insulin-like peptide 5 (INSL5) has recently been discovered as only the second orexigenic gut hormone after ghrelin. As we have previously reported,INSL5 is extremely difficult to assemble and oxidize into its two-chain three-disulfide structure. The focus of this study was to generate structure-activity relationships (SARs) of INSL5 and use it to develop a potent and simpler INSL5 mimetic with RXFP4 agonist activity. A series of human and mouse INSL5 (hINSL5/mINSL5) analogues were designed and chemically synthesized, resulting in a chimeric INSL5 analogue exhibiting more than 10-fold higher potency (0.35 nM) at human RXFP4 compared with native hINSL5 (4.57 nM). The SAR study also identified a key residue (K(A15)) in the A-chain of mINSL5 that contributes to improved RXFP4 affinity and potency of mINSL5 compared with hINSL5. This knowledge ultimately led us to engineer a minimized hINSL5 mimetic agonist that retains native hINSL5-like RXFP4 affinity and potency at human RXFP4. This minimized analogue was synthesized in 17.5-fold higher yield and in less time compared with hINSL5.
Patil NA, Hughes RA, Rosengren KJ, et al. Engineering of a Novel Simplified Human Insulin-Like Peptide 5 Agonist. J Med Chem. 2016;59(5):2118-25.
OBJECTIVE: Insulin-like peptide 5 (INSL5) is a recently identified gut hormone that is produced predominantly by L-cells in the colon, but its function is unclear. We have previously shown that colonic expression of the gene for the L-cell hormone GLP-1 is high in mice that lack a microbiota and thus have energy-deprived colonocytes. Our aim was to investigate if energy deficiency also affected colonic Insl5 expression and to identify a potential role of INSL5.
METHODS: We analyzed colonic Insl5 expression in germ-free (GF), conventionally raised (CONV-R), conventionalized (CONV-D) and antibiotic-treated mice, and also assessed the effect of dietary changes on colonic Insl5 expression. In addition, we characterized the metabolic phenotype of Insl5-/- mice.
RESULTS: We showed that colonic Insl5expression was higher in GF and antibiotic-treated mice than in CONV-R mice, whereasInsl5 expression in the brain was higher in CONV-R versus GF mice. We also observed that colonic Insl5 expression was suppressed by increasing the energy supply in GF mice by colonization or high-fat feeding. We did not observe any differences in food intake, gut transit or oral glucose tolerance betweenInsl5-/- and wild-type mice. However, we showed impaired intraperitoneal glucose tolerance in Insl5-/- mice. We also observed improved insulin tolerance and reduced hepatic glucose production in Insl5-/- mice. Conclusions: We have shown that colonic Insl5expression is regulated by the gut microbiota and energy availability. We propose that INSL5 is a hormone that could play a role in promoting hepatic glucose production during periods of energy deprivation.
This publication used an INSL5 antibody (cat#G-035-40) from Phoenix Pharmaceuticals.
Lee YS, De vadder F, Tremaroli V, Wichmann A, Mithieux G, Bäckhed F. Insulin-like peptide 5 is a microbially regulated peptide that promotes hepatic glucose production. Mol Metab. 2016;5(4):263-70.
BACKGROUND AND PURPOSE: Using an in-house bioinformatics programme, we identified and synthesized a novel nonapeptide, H-Pro-Pro-Thr-Thr-Thr-Lys-Phe-Ala-Ala-OH. Here, we have studied its biological activity, in vitro and in vivo, and have identified its target in the brain.
EXPERIMENTAL APPROACH: The affinity of the peptide was characterized using purified whole brain and striatal membranes from guinea pigs and rats . Its effect on behaviour in rats following intra-striatal injection of the peptide was investigated. A photoaffinity UV cross-linking approach combined with subsequent affinity purification of the ligand covalently bound to its receptor allowed identification of its target.
KEY RESULTS: The peptide bound with high affinity to a single class of binding sites, specifically localized in the striatum and substantia nigra of brains from guinea pigs and rats. When injected within the striatum of rats, the peptide stimulated in vitro and in vivo dopamine release and induced dopamine-like motor effects. We purified the target of the peptide, a ~151 kDa protein that was identified by MS/MS as angiotensin converting enzyme (ACE I). Therefore, we decided to name the peptide acein.
CONCLUSION AND IMPLICATIONS: The synthetic nonapeptide acein interacted with high affinity with brain membrane-bound ACE. This interaction occurs at a different site from the active site involved in the well-known peptidase activity, without modifying the peptidase activity. Acein, in vitro and in vivo, significantly increased stimulated release of dopamine from the brain. These results suggest a more important role for brain ACE than initially suspected.
Neasta J, Valmalle C, Coyne AC, et al. The novel nonapeptide acein targets angiotensin converting enzyme in the brain and induces dopamine release. Br J Pharmacol. 2016;173(8):1314-28.
Relaxin family peptide 3 receptors (RXFP3) are activated by H3-relaxin to inhibit forskolin-stimulated cAMP accumulation and stimulate extracellular signal-regulated kinase (ERK) 1/2 phosphorylation. In this study, we sought to identify novel signaling pathways coupled to RXFP3 and to investigate whether other members of the relaxin peptide family activated these pathways. Two patterns of signaling were observed in RXFP3-expressing Chinese hamster ovary (CHO)-K1 and human embryonic kidney (HEK)-293 cells (CHO-RXFP3 and HEK-RXFP3) and murine septal neuron SN56 cell lines: 1) strong inhibition of forskolin-stimulated cAMP accumulation, ERK1/2 activation and nuclear factor (NF)-kappaB reporter gene activation in cells stimulated with H3 relaxin, with weaker activity observed for H2 relaxin, porcine relaxin, or insulin-like peptide (INSL) 3 and 2) strong stimulation of activator protein (AP)-1 reporter genes by H2 relaxin, with weaker activation observed with H3 or porcine relaxin. Two distinct ligand binding sites were identified on RXFP3-expressing cells using two different radioligands. (125)I-INSL5 A-chain/relaxin-3 B-chain chimera bound with high affinity to the RXFP3-expressing cells with competition by H3 relaxin or a H3 relaxin B-chain dimeric peptide, consistent with previous reports. Binding studies with (125)I-H2 relaxin revealed a distinct binding site with potent competition observed with H2 relaxin, H3 relaxin, or INSL3 and weaker competition with porcine relaxin. Thus H3 relaxin potently activates all signaling pathways coupled to RXFP3, whereas H2 relaxin is an AP-1-biased ligand relative to H3 relaxin.
Van der westhuizen ET, Christopoulos A, Sexton PM, Wade JD, Summers RJ. H2 relaxin is a biased ligand relative to H3 relaxin at the relaxin family peptide receptor 3 (RXFP3). Mol Pharmacol. 2010;77(5):759-72.
Relaxin-3 and its endogenous receptor RXFP3 are involved in fundamental neurological signalling pathways, such as learning and memory, stress, feeding and addictive behaviour. Consequently, this signalling system has emerged as an attractive drug target. Development of leads targeting RXFP3 relies on assays for screening and ligand optimization. Here, we present the synthesis and in vitro characterization of a fluorescent europium-labelled antagonist of RXFP3. This ligand represents a cheap and safe but powerful tool for future mechanistic and cell-based receptor-ligand interaction studies of the RXFP3 receptor.
Haugaard-kedström LM, Wong LL, Bathgate RA, Rosengren KJ. Synthesis and pharmacological characterization of a europium-labelled single-chain antagonist for binding studies of the relaxin-3 receptor RXFP3. Amino Acids. 2015;47(6):1267-71.
Relaxin-like peptides (RLN/INSL) play diverse roles in reproductive and neuroendocrine processes in placental mammals and are functionally associated with two distinct types of receptors (RXFP) for each respective function. The diversification of RLN/INSL and RXFP gene families in vertebrates was predominantly driven by whole genome duplications (2R and 3R). Teleosts preferentially retained duplicates of genes putatively involved in neuroendocrine regulation, harboring a total of 10-11 receptors and 6 ligand genes, while most mammals have equal numbers of ligands and receptors. To date, the ligand-receptor relationships of teleost Rln/Insl peptides and their receptors have largely remained unexplored. Here, we use selection analyses based on sequence data from 5 teleosts and qPCR expression data from zebrafish to explore possible ligand-receptor pairings in teleosts. We find support for the hypothesis that, with the exception of RLN, which has undergone strong positive selection in mammalian lineages, the ligand and receptor genes shared between mammals and teleosts appear to have similar pairings. On the other hand, the teleost-specific receptors show evidence of subfunctionalization. Overall, this study underscores the complexity of RLN/INSL and RXFP ligand-receptor interactions in teleosts and establishes theoretical background for further experimental work in nonmammals.
Good S, Yegorov S, Martijn J, Franck J, Bogerd J. New insights into ligand-receptor pairing and coevolution of relaxin family peptides and their receptors in teleosts. Int J Evol Biol. 2012;2012:310278.
Relaxin-3 is a two-chain disulfide-rich peptide that is the ancestral member of the relaxin peptide family and, together with its G protein-coupled receptor RXFP3, is highly expressed in the brain. Strong evolutionary conservation of relaxin-3 suggests a critical biological function and recent studies have demonstrated modulation of sensory, neuroendocrine, metabolic, and cognitive systems. However, detailed studies of central relaxin-3-RXFP3 signaling have until now been severely hampered by the lack of a readily available high-affinity antagonist for RXFP3. Previous studies have utilized a complex two-chain chimeric relaxin peptide, R3(B?23-27)R/I5, in which a truncated relaxin-3 B-chain carrying an additional C-terminal Arg residue was combined with the insulin-like peptide 5 (INSL5) A-chain. In this study we demonstrate that, by replacing the native Cys in this truncatedrelaxin-3 B-chain with Ser, a single-chain linear peptide of 23 amino acids that retains high-affinity antagonism for RXFP3 can be achieved. In vivo studies demonstrate that this peptide, R3 B1-22R, antagonized relaxin-3/RXFP3 induced increases in feeding in rats after intracerebroventricular injection. Thus, R3 B1-22R represents an excellent tool for biological studies probing relaxin pharmacology and a lead molecule for the development of synthetically tractable, single-chain RXFP3 modulators for clinical use.
Haugaard-kedström LM, Shabanpoor F, Hossain MA, et al. Design, synthesis, and characterization of a single-chain peptide antagonist for the relaxin-3 receptor RXFP3. J Am Chem Soc. 2011;133(13):4965-74.
Relaxin family peptide 3 receptors (RXFP3) are activated by H3-relaxin to inhibit forskolin-stimulated cAMP accumulation and stimulate extracellular signal-regulated kinase (ERK) 1/2 phosphorylation. In this study, we sought to identify novel signaling pathways coupled to RXFP3 and to investigate whether other members of the relaxin peptide family activated these pathways. Two patterns of signaling were observed in RXFP3-expressing Chinese hamster ovary (CHO)-K1 and human embryonic kidney (HEK)-293 cells (CHO-RXFP3 and HEK-RXFP3) and murine septal neuron SN56 cell lines: 1) strong inhibition of forskolin-stimulated cAMP accumulation, ERK1/2 activation and nuclear factor (NF)-kappaB reporter gene activation in cells stimulated with H3 relaxin, with weaker activity observed for H2 relaxin, porcine relaxin, or insulin-like peptide (INSL) 3 and 2) strong stimulation of activator protein (AP)-1 reporter genes by H2 relaxin, with weaker activation observed with H3 or porcine relaxin. Two distinct ligand binding sites were identified on RXFP3-expressing cells using two different radioligands. (125)I-INSL5 A-chain/relaxin-3 B-chain chimera bound with high affinity to the RXFP3-expressing cells with competition by H3 relaxin or a H3 relaxin B-chain dimeric peptide, consistent with previous reports. Binding studies with (125)I-H2 relaxin revealed a distinct binding site with potent competition observed with H2 relaxin, H3 relaxin, or INSL3 and weaker competition with porcine relaxin. Thus H3 relaxin potently activates all signaling pathways coupled to RXFP3, whereas H2 relaxin is an AP-1-biased ligand relative to H3 relaxin.
Both relaxin-3 and its receptor (GPCR135) are expressed predominantly in brain regions known to play important roles in processing sensory signals. Recent studies have shown that relaxin-3 is involved in the regulation of stress and feeding behaviors. The mechanisms underlying the involvement of relaxin-3/GPCR135 in the regulation of stress, feeding, and other potential functions remain to be studied. Because relaxin-3 also activates the relaxin receptor (LGR7), which is also expressed in the brain, selective GPCR135 agonists and antagonists are crucial to the study of the physiological functions of relaxin-3 and GPCR135 in vivo. Previously, we reported the creation of a selective GPCR135 agonist (a chimeric relaxin-3/INSL5 peptide designated R3/I5). In this report, we describe the creation of a high affinity antagonist for GPCR135 and GPCR142 over LGR7. This GPCR135 antagonist, R3(BDelta23–27)R/I5, consists of the relaxin-3 B-chain with a replacement of Gly23 to Arg, a truncation at the C terminus (Gly24-Trp27 deleted), and the A-chain of INSL5. In vitro pharmacological studies showed that R3(BDelta23–27)R/I5 binds to human GPCR135 (IC50 = 0.67 nM) and GPCR142 (IC50 = 2.29 nM) with high affinity and is a potent functional GPCR135 antagonist (pA2 = 9.15) but is not a human LGR7 ligand. Furthermore, R3(BDelta23–27)R/I5 had a similar binding profile at the rat GPCR135 receptor (IC50 = 0.25 nM, pA2 = 9.6) and lacked affinity for the rat LGR7 receptor. When administered to rats intracerebroventricularly, R3(BDelta23–27)R/I5 blocked food intake induced by the GPCR135 selective agonist R3/I5. Thus, R3(BDelta23–27)R/I5 should prove a useful tool for the further delineation of the functions of the relaxin-3/GPCR135 system.
Haruta M, Sasai Y, Kawasaki H, et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol Vis Sci. 2004;45(3):1020-5.
Relaxin-3 (R3) is the latest member of the insulin (INSL) superfamily, which is composed of peptides with diverse sequences held together by characteristic disulfide links connecting A and B peptide-Chains. R3 has nearly exclusive expression in the brainstem and was demonstrated to be an additional ligand for LGR7. We recently identified R3 as a ligand for two orphan G-protein coupled receptors, GPCR135 and GPCR142. The predominant brain expression for both R3 and GPCR135, coupled with their high affinity interaction, strongly suggests that R3 is the endogenous ligand for GPCR135. Both R3 and GPCR135 from different species are highly conserved from genetic sequences to in vitro pharmacology. By contrast, GPCR142 is a pseudogene in the rat, and the mouse gene is less conserved with human GPCR142, suggesting that GPCR142 may have a diminished role as a receptor for R3 in the rodent. In addition, the tissue expression pattern of GPCR142, which is primarily in peripheral tissue, is drastically different from that of R3, suggesting that GPCR142 may have an endogenous ligand other than R3. Sequence analysis amongINSL/relaxin family members shows that INSL5 is the closest member of R3. We were able to demonstrate that INSL-5 is an agonist for GPCR142 but not for GPCR135. We also showed that the mRNA expression pattern of INSL-5 overlaps with that of GPCR142. By substituting the A-Chain of R3 with the A-Chain of INSL-5, we devised a chimeric peptide (R3/I5) that is about 1000-fold selective for GPCR135 and GPCR142 over LGR7. Autoradiographic distribution of GPCR135 binding sites using [125I]R3/I5 in rat brain shows that GPCR135 receptor is most prominent in areas known for the processing of sensory signals.
Liu C, Bonaventure P, Sutton SW, et al. Recent progress in relaxin-3-related research. Ann N Y Acad Sci. 2005;1041:47-60.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 028-88 | GLP-1(1-12)[D-Ala2]-Dynophin A Chimera | 100 μg | $395 |
| 028-90 | Exendin-4(1-27)-Leu-Enkephalin [D-Ala2,29]-Amide Chimera | 100 μg | $315 |
| 036-68 | INSL5 (Human)-A13 / RXFP4 Agonist (Human) | 20 µg | $175 |
| 036-72 | INSL5 (Human)-A13NR / RXFP4 Antagonist (Human) | 20 µg | $175 |
| 028-98 | GLP-1-PYY Chimera GEP12 | 100 μg | $395 |
| 028-97 | GLP-1-PYY Chimera GEP44 | 100 μg | $395 |
| 036-51 | RXFP3 specific agonist [G(B24)S]R3/I5 | 100 µg | $428 |
| 028-89 | SA10SC-RLX / Compound 1 | 100 μg | $368 |
Social Network Confirmation