Abstract: Deterioration of insulin secretion and pancreatic beta-cell mass by inflammatory attacks is one of the main pathophysiological features of type 2 diabetes (T2D). Therefore, preserving beta-cell mass and stimulating insulin secretion only in response to glucose for avoiding the hypoglycemia risks, are the most state-of-the-art option for the treatment of T2D. In this study we tested two correlated hypothesis that 1/ the endogenous peptide released from sortilin, known as PE, that stimulates insulin secretion only in response to glucose, protects beta-cells against death induced by cytokines, and 2/ Spadin and Mini-Spadin, two synthetic peptides derived from PE, that mimic the effects of PE in insulin secretion, also provide beneficial effect on beta-cells survival. We show that PE and its derivatives by inducing a rise of intracellular calcium concentration by depolarizing the membrane protect beta-cells against death induced by Interleukin-1β. Using biochemical, confocal imaging and cell biology techniques, we reveal that the protective effects of PE and its derivatives rely on the activation of the CaM-Kinase pathway, and on the phosphorylation and activation of the transcription factor CREB. In addition, Mini-Spadin promotes beta-cell proliferation, suggesting its possible regenerative effect. This study highlights new possible roles of PE in pancreatic beta-cell survival and its derivatives as pharmacological tools against diabetes.
Daziano G, Blondeau N, Béraud-Dufour S, et al. Sortilin-derived peptides promote pancreatic beta-cell survival through CREB signaling pathway. Pharmacological Research. 2021;167:105539.
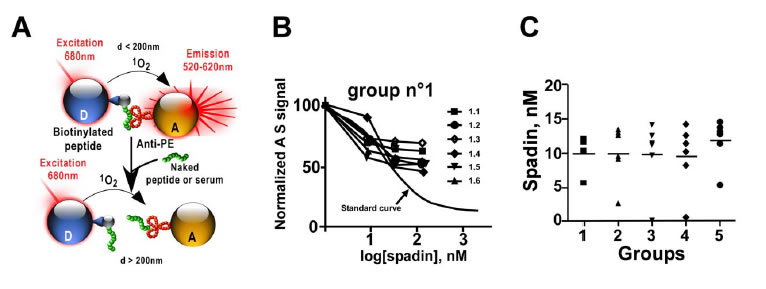
Current antidepressant treatments are inadequate for many individuals, and when they are effective, they require several weeks of administration before a therapeutic effect can be observed. Improving the treatment of depression is challenging. Recently, the two-pore domain potassium channel TREK-1 has been identified as a new target in depression, and its antagonists might become effective antidepressants. In mice, deletion of the TREK-1 gene results in a depression-resistant phenotype that mimics antidepressant treatments. Here, we validate in mice the antidepressant effects of spadin, a secreted peptide derived from the propeptide generated by the maturation of the neurotensin receptor 3 (NTSR3/Sortilin) and acting through TREK-1 inhibition. NTSR3/Sortilin interacted with the TREK-1 channel, as shown by immunoprecipitation of TREK-1 and NTSR3/Sortilin from COS-7 cells and cortical neurons co-expressing both proteins. TREK-1 and NTSR3/Sortilin were colocalized in mouse cortical neurons. Spadin bound specifically to TREK-1 with an affinity of 10 nM. Electrophysiological studies showed that spadin efficiently blocked the TREK-1 activity in COS-7 cells, cultured hippocampal pyramidal neurons, and CA3 hippocampal neurons in brain slices. Spadin also induced in vivo an increase of the 5-HT neuron firing rate in the Dorsal Raphe Nucleus. In five behavioral tests predicting an antidepressant response, spadin-treated mice showed a resistance to depression as found in TREK-1 deficient mice. More importantly, an intravenous 4-d treatment with spadin not only induced a strong antidepressant effect but also enhanced hippocampal phosphorylation of CREB protein and neurogenesis, considered to be key markers of antidepressant action after chronic treatment with selective serotonin reuptake inhibitors. This work also shows the development of a reliable method for dosing the propeptide in serum of mice by using AlphaScreen technology. These findings point out spadin as a putative antidepressant of new generation with a rapid onset of action. Spadin can be regarded as the first natural antidepressant peptide identified. It corresponds to a new concept to address the treatment of depression.
Mazella J, Pétrault O, Lucas G, et al. Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: a new concept in the antidepressant drug design. PLoS Biol. 2010;8(4):e1000355.
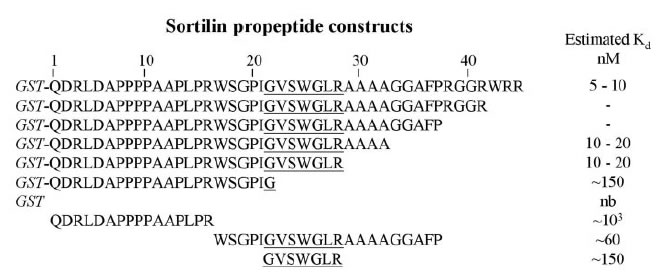
A Vps10p domain makes up the entire luminal part of Sortilin, and this type of domain is the hallmark of a new family of neuronal receptors that target a variety of ligands, including neurotrophins and neuropeptides. We have shown that two structural features of the Vps10p domain, the N-terminal propeptide and the C-terminal segment of ten conserved cysteines (10CC), are key elements in the function of Sortilin. The propeptide has two functions. (i) It binds the mature part of Sortilin and prevents ligands in the biosynthetic pathway from binding to the uncleaved proreceptor, and (ii) it facilitates receptor transport in early Golgi compartments by a mechanism that does not depend on its ability to prevent ligand binding. In contrast, other Vps10p domain receptors, such as SorLA and SorCS3, do not need their propeptide for normal and swift processing. The 10CC segment constitutes an exchangeable module containing five conserved disulfide bridges, and using module-shuffling and truncations, we have shown that the 10CC segment is a major ligand-binding region in Sortilin.
Westergaard UB, Sørensen ES, Hermey G, et al. Functional organization of the sortilin Vps10p domain. J Biol Chem. 2004;279(48):50221-9.
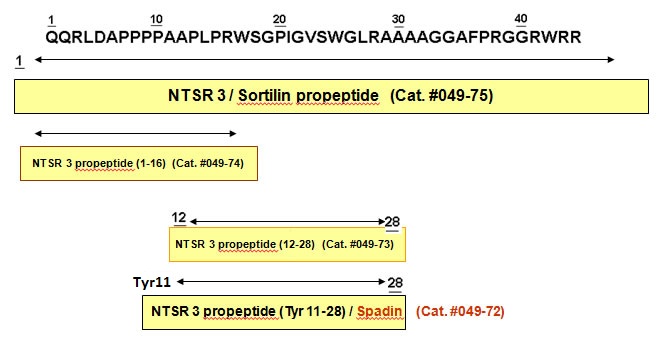
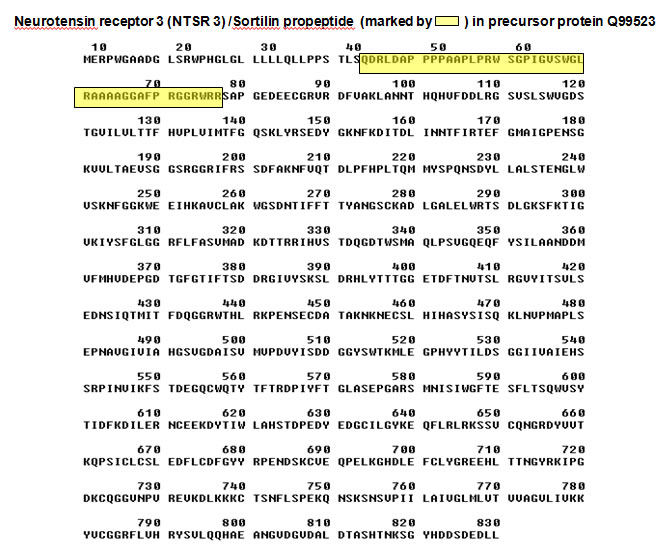
We recently reported the isolation and sequencing of sortilin, a new putative sorting receptor that binds receptor-associated protein (RAP). The luminal N-terminus of sortilin comprises a consensus sequence for cleavage by furin, R41WRR44, which precedes a truncation originally found in sortilin isolated from human brain. We now show that the truncation results from cellular processing. Sortilin is synthesized as a proform which, in late Golgi compartments, is converted to the mature receptor by furin-mediated cleavage of a 44 residue N-terminal propeptide. We further demonstrate that the propeptide exhibits pH-dependent high affinity binding to fully processed sortilin, that the binding is competed for by RAP and the newly discovered sortilin ligand neurotensin, and that prevention of propeptide cleavage essentially prevents binding of RAP and neurotensin. The findings evidence that the propeptide sterically hinders ligands from gaining access to overlapping binding sites in prosortilin, and that cleavage and release of the propeptide preconditions sortilin for full functional activity. Although proteolytic processing is involved in the maturation of several receptors, the described exposure of previously concealed ligand-binding sites after furin-mediated cleavage of propeptide represents a novel mechanism in receptor activation.
Munck petersen C, Nielsen MS, Jacobsen C, et al. Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J. 1999;18(3):595-604.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 049-72 | Spadin (Human) | 200 µg | $152 |
| 049-76 | Mini-Spadin (Human) | 500 µg | $202 |
| 049-74 | NTSR 3 (1-16) / Sortilin Propeptide (1-16) (Human) | 200 µg | $152 |
| 049-73 | NTSR 3 (12-28) / Sortilin Propeptide (12-28) (Human) | 200 µg | $140 |
| 049-75 | NTSR 3 / Sortilin Propeptide (Human) | 100 µg | $444 |
Social Network Confirmation