While common in viral infections and neoplasia, spontaneous cell-cell fusion, or syncytialization, is quite restricted in healthy tissues. Such fusion is essential to human placental development, where interactions between trophoblast-specific human endogenous retroviral (HERV) envelope proteins, called syncytins, and their widely-distributed cell surface receptors are centrally involved. We have identified the first host cell-encoded protein that inhibits cell fusion in mammals. Like the syncytins, this protein, called suppressyn, is HERV-derived, placenta-specific and well-conserved over simian evolution. In vitro, suppressyn binds to the syn1 receptor and inhibits syn1-, but not syn2-mediated trophoblast syncytialization. Suppressyn knock-down promotes cell-cell fusion in trophoblast cells and cell-associated and secreted suppressyn binds to the syn1 receptor, ASCT2. Identification of the first host cell-encoded inhibitor of mammalian cell fusion may encourage improved understanding of cell fusion mechanisms, of placental morphogenesis and of diseases resulting from abnormal cell fusion.
Sugimoto J, Sugimoto M, Bernstein H, Jinno Y, Schust D. A novel human endogenous retroviral protein inhibits cell-cell fusion. Sci Rep. 2013;3:1462.
Up to 8% of the human genome is of retroviral origin. These stably integrated retroviral sequences that characterize the human endogenous retrovirus (HERV) arose from retroviral infections that occurred more than 25 million years ago. The host and the retrovirus have subsequently coevolved as retrovirally derived genetic material is propagated in a Mendelian fashion. Although most HERV sequences are silenced, several have been described that are functional. The effects of some HERV-derived products are linked to human disease; others appear essential to human organ function. The human placenta, unique in its active expression of retroviral sequences that are not expressed in other tissues, may hold the key to an improved understanding of the functional significance of HERVs. In this review, we discuss the contribution of retroelements, particularly HERVs, to placental function and dysfunction. We describe fusogenic and immunosuppressive HERV activities and emphasize epigenetic regulation of retroelement expression.
Sugimoto J, Schust DJ. Review: human endogenous retroviruses and the placenta. Reprod Sci. 2009;16(11):1023-33.
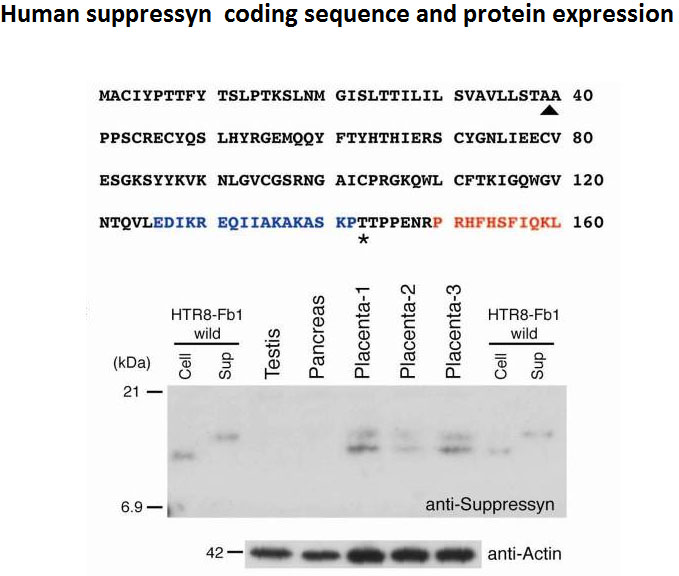
We previously investigated tissue specificity and temporal changes in expression of five human endogenous retroviruses (HERVs), including syncytin/ERVWE1 and syncytin 2. In the current study, we examined the cellular localization and quantified the transcripts of five HERVs, syncytin, syncytin 2, HERV-H7/F(XA34), HERV-Fb1, and HERV-HML6-c14, in order to elucidate their physiological and etiological roles in the placenta and in pregnancy-induced hypertension (PIH), respectively. In situ hybridization revealed trophoblast-specific transcription of all five HERVs. Consistent with a previous immunohistochemical analysis, syncytin 2 transcripts were detected only in cytotrophoblasts. All the HERVs except HERV-HML6-c14 (HML6-c14) were predominantly localized in the cytoplasm of syncytiotrophoblasts and/or cytotrophoblasts. Quantitative analysis showed that transcriptional levels of these four HERVs were lower in placentas obtained from pregnant women with PIH (n=22) than in those from normotensive pregnant women (n=87) and that the differences were statistically significant (p=0.001, 0.01, <0.001, and 0.04 for syncytin, syncytin 2, HERV-H7/F(XA34), and HERV-Fb1, respectively). In contrast to the other HERVs, HML6-c14 transcripts localized to the nucleus and the average transcriptional level of HML6-c14 was higher in PIH placentas than in control placentas from normotensive pregnant women, although the differences did not reach significance (p=0.19). These results suggest that placenta-specific HERVs may have some function in the human placenta and that their reduced expression in PIH placentas is not merely a secondary effect derived from the pathology of PIH placentas.
Kudaka W, Oda T, Jinno Y, Yoshimi N, Aoki Y. Cellular localization of placenta-specific human endogenous retrovirus (HERV) transcripts and their possible implication in pregnancy-induced hypertension. Placenta. 2008;29(3):282-9.
The evolutional and biological roles of human endogenous retroviruses (HERVs) are less recognized compared to those of L1. In the present study, we focused on the transcriptional activity of HERVs in normal human tissues and found five HERV loci that are actively expressed in normal tissues. All but one showed tissue specificity of expression: one was expressed in stomach and small intestine and three were in placenta. We subsequently examined by TaqMan-based RT-PCR assays the temporal expression profiles of the three placenta-specific HERVs along with syncytin and syncytin2 and observed three patterns. Syncytin and HERV-Fb showed almost constant expression through gestations. Syncytin 2 gradually decreased as pregnancy proceeded. In contrast, expression from the HERV-H/F and HERV-K(HML-6) loci increased remarkably in term placentas. Term placentas in general showed larger interindividual differences in HERV expression levels. Our results suggest that HERVs might have more diverse effects than currently thought.
Blomberg J, Ushameckis D, Jern P. Evolutionary Aspects of Human Endogenous Retroviral Sequences (HERVs) and Disease. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6235/
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
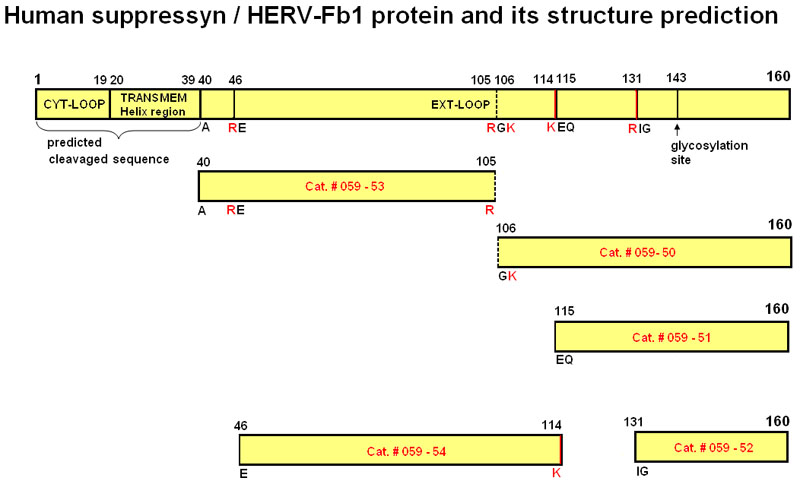
| 059-50 | Suppressyn (106-160) / HERV-Fb1 (106-160) (Human) | 100 µg | $382 |
| 059-51 | Suppressyn (115-160) / HERV-Fb1 (115-160) (Human) | 100 µg | $356 |
| 059-52 | Suppressyn (131-160) / HERV-Fb1 (131-160) (Human) | 100 µg | $317 |
| H-059-52 | Suppressyn (131-160) / HERV-Fb1 (131-160) (Human) - Antibody | 50 µl | $562 |
| 059-53 | Suppressyn (40-105) / HERV-Fb1 (40-105) (Human) | 100 µg | $444 |
| 059-54 | Suppressyn (46-114) / HERV-Fb1 (46-114) (Human) | 100 µg | $444 |
Social Network Confirmation