
RMP16, a recombinant TNF α-derived polypeptide comprising a specific human serum albumin (HSA)-binding 7-mer peptide identified by phage display screening (WQRPSSW), a cleavage peptide for Factor Xa (IEGR), and a 20-amino acid bioactive peptide P16 (TNF α segment including amino acid residues 75–94), was prepared by gene-engineering technology. RMP16 showed prolonged half-life, 13.11 hours in mice (half-lives of P16 and TNF α are 5.77 and 29.0?minutes, respectively), and obviously higher receptor selectivity for TNFRI than TNF ?. RMP16 had significant inhibition effects for multiple tumor cells, especially prostate cancer Du145 cells, and human vascular endothelial cells but not for human mammary non-tumorigenic epithelial cells. RMP16 can more effectively induce apoptosis and inhibit proliferation for DU145 cells than P16 and TNF α via the caspase-dependent apoptosis pathway and G0/G1 cell cycle arrest. In nude mice with transplanted tumor of DU145 cells, RMP16 significantly induced apoptosis and necrosis of tumor tissues but causing less side effects, and tumor inhibitory rate reached nearly 80%, furthermore, RMP16 can potently inhibit tumor angiogenesis and neovascularization. These findings suggest that RMP16 may represent a promising long-lasting antitumor therapeutic peptide with less TNF α-induced toxicity.
Ma Y, Zhao S, Shen S, et al. A novel recombinant slow-release TNF α-derived peptide effectively inhibits tumor growth and angiogensis. Sci Rep. 2015;5:13595.
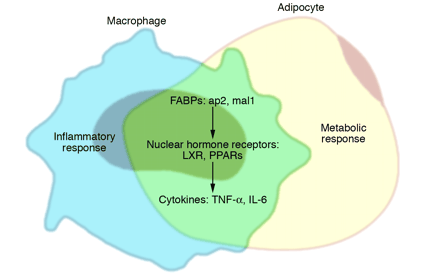
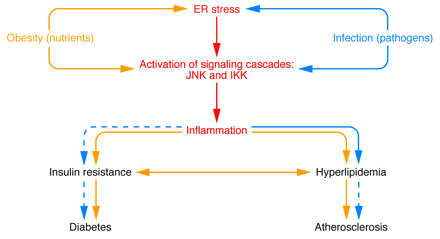
Over the last decade, an abundance of evidence has emerged demonstrating a close link between metabolism and immunity. It is now clear that obesity is associated with a state of chronic low-level inflammation. In this article, we discuss the molecular and cellular underpinnings of obesity-induced inflammation and the signaling pathways at the intersection of metabolism and inflammation that contribute to diabetes. We also consider mechanisms through which the inflammatory response may be initiated and discuss the reasons for the inflammatory response in obesity. We put forth for consideration some hypotheses regarding important unanswered questions in the field and suggest a model for the integration of inflammatory and metabolic pathways in metabolic disease.
Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111-9.
Over the last decade, an abundance of evidence has emerged demonstrating a close link between metabolism and immunity. It is now clear that obesity is associated with a state of chronic low-level inflammation. In this article, we discuss the molecular and cellular underpinnings of obesity-induced inflammation and the signaling pathways at the intersection of metabolism and inflammation that contribute to diabetes. We also consider mechanisms through which the inflammatory response may be initiated and discuss the reasons for the inflammatory response in obesity. We put forth for consideration some hypotheses regarding important unanswered questions in the field and suggest a model for the integration of inflammatory and metabolic pathways in metabolic disease.
Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. Journal of Clinical Investigation. 2005;115(5):1111-1119. doi:10.1172/JCI200525102.
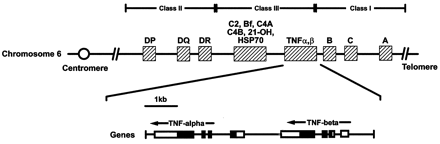
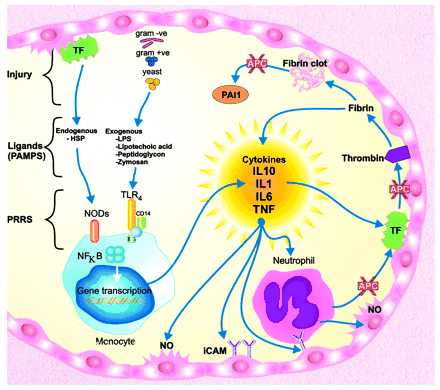
Genetic epidemiologic studies suggest a strong genetic influence on the outcome from sepsis, and genetics may explain the wide variation in the individual response to infection that has long puzzled clinicians. Several candidate genes have been identified as important in the inflammatory response and investigated in case-controlled studies, including the tumor necrosis factor (TNF)-alpha and TNF-beta genes, positioned next to each other within the cluster of human leukocyte antigen class III genes on chromosome 6. Other candidate genes for sepsis and septic shock include the interleukin (IL)-1 receptor antagonist gene, the heat shock protein gene, the IL-6 gene, the IL-10 gene, the CD-14 gene, the Toll-like receptor (TLR)-4 gene, and the TLR-2 gene, to name a few. In this review, we summarize the evidence for a genetic susceptibility to development of sepsis and death from sepsis, discuss design of clinical genetics studies relevant to the study of complex disorders, consider the candidate genes likely to be involved in the pathogenesis of sepsis, and discuss the potential for targeted therapy of sepsis and septic shock based on genetic variability.
Holmes CL, Russell JA, Walley KR. Genetic polymorphisms in sepsis and septic shock: role in prognosis and potential for therapy. Chest. 2003;124(3):1103-15.
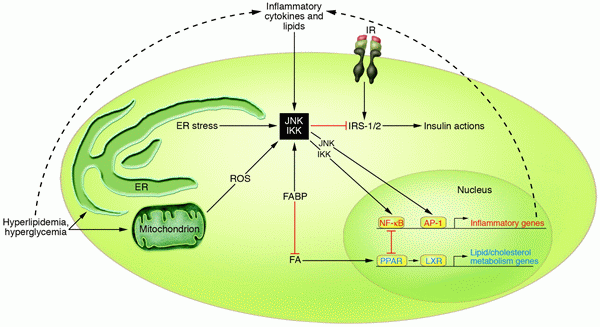
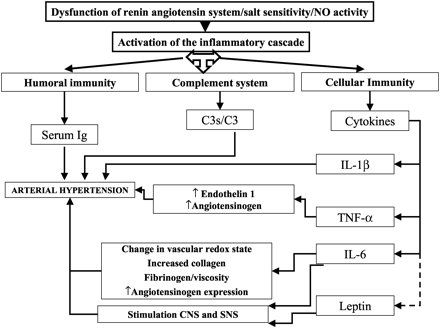
Insulin resistance is increasingly recognized as a chronic, low-level, inflammatory state. Hyperinsulinemia and insulin action were initially proposed as the common preceding factors of hypertension, low high-density lipoprotein cholesterol, hypertriglyceridemia, abdominal obesity, and altered glucose tolerance, linking all these abnormalities to the development of coronary heart disease. The similarities of insulin resistance with another inflammatory state, atherosclerosis, have been described only in the last few decades. Atherosclerosis and insulin resistance share similar pathophysiological mechanisms, mainly due to the actions of the two major proinflammatory cytokines, TNF-alpha and IL-6. Genetic predisposition to increased transcription rates of these cytokines is associated with metabolic derangement and simultaneously with coronary heart disease. Dysregulation of the inflammatory axis predicts the development of insulin resistance and type 2 diabetes mellitus. The knowledge of how interactions between metabolic and inflammatory pathways occur will be useful in future therapeutic strategies. The effective administration of antiinflammatory agents in the treatment of insulin resistance and atherosclerosis is only the beginning of a promising approach in the management of these syndromes.
Fernández-real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24(3):278-301.
A periurban outbreak of visceral leishmaniasis (VL) caused by the protozoan Leishmania chagasi is ongoing outside Natal, northeast Brazil. Manifestations range from asymptomatic infection to disseminated visceral disease. Literature reports suggest that both genetic and environmental factors influence the outcome of infection. Due to the association of the tumor necrosis factor (TNF) locus with other infectious diseases, we examined whether polymorphic alleles at this locus are associated with the outcome of L. chagasi infection. Neighborhoods with ongoing transmission were identified through patients admitted to local hospitals. Altogether, 1,024 individuals from 183 families were classified with the following disease phenotypes: (i) symptomatic VL, (ii) asymptomatic infection (positive delayed-type hypersensitivity [DTH+]), or (iii) no evidence of infection (DTH-). Genotypes were determined at a microsatellite marker (MSM) upstream of the TNFB gene encoding TNF-beta and at a restriction fragment length polymorphism (RFLP) at position -307 in the promoter of the TNFA gene encoding TNF-alpha. Analyses showed that the distribution of TNFA RFLP alleles (TNF1 and TNF2) and the TNF MSM alleles (TNFa1 to TNFa15) differed between individuals with VL and those with DTH+ phenotypes. TNF1 was transmitted more frequently than expected from heterozygous parents to DTH+ offspring (P = 0.0006), and haplotypes containing TNF2 were associated with symptomatic VL (P = 0.0265, transmission disequilibrium test). Resting serum TNF-alpha levels were higher in TNF1/2 heterozygotes than in TNF1/1 homozygotes (P < 0.05). These data led us to hypothesize that an individual's genotype at the TNF locus may be associated with whether he or she develops asymptomatic or symptomatic disease after L. chagasi infection. The results preliminarily suggest that this may be the case, and follow-up with larger populations is needed for verification.
Karplus TM, Jeronimo SM, Chang H, et al. Association between the tumor necrosis factor locus and the clinical outcome of Leishmania chagasi infection. Infect Immun. 2002;70(12):6919-25.
In recent years, much has been written about the importance of diabetes mellitus, both as a cause of widespread morbidity and mortality and in terms of the resultant overwhelming health care costs. In an analysis of worldwide diabetes in 1994, the World Health Organization reported that the age-standardized prevalence in European populations varied from 3% to 10%, while more restricted populations demonstrated prevalence of up to 50%. Over the same period, approximately 10.2 million people with diagnosed diabetes mellitus resided in the US, but another 5.4 million individuals with diabetes went undiagnosed. Perhaps even more impressive, these numbers have been estimated to represent as much as a 50% increase over the prevalence in equivalent populations during the previous decade. In 1997, health care expenditures attributable to diabetes in the US were estimated to be $98 billion. Undoubtedly, this is due not only to the widespread prevalence of the disease, but also to its chronic nature and disabling complications, affecting cardiovascular, renal, visual, and neurological function. Thus, perhaps the lack of an even more intense research effort into the pathophysiology of diabetes can only be attributed to the difficulty inherent in making progress in the study of this complex, multisystem disease. For these reasons, it is particularly important that in two recent articles — one appearing in the current issue of the journal Science and the other a recent issue of the JCI — Steven Shoelson, Gerald Shulman, and their colleagues present a new hypothesis, which not only purports to explain the insulin resistance of type 2 diabetes mellitus but also offers a clear basis for the development of novel therapeutics.
Over 90% of diabetes mellitus is accounted for by what is now called the type 2 variant. Unlike type 1 diabetes, for which there is a reasonable consensus that the disease results from autoimmune destruction of insulin-secreting pancreatic β cells, the etiology of type 2 diabetes remains a bit uncertain. Most investigators and clinicians agree that genetic and environmental factors contribute and that obesity is a frequent if not essential antecedent of the disease. Perhaps the most heated debate among diabetes researchers has concerned the nature of the primary inciting metabolic event, that is, whether it represents a disturbance in the normal pattern of insulin secretion or abnormalities in the action of insulin in peripheral tissues. Experiments in which defects in insulin secretion or action have been selectively introduced into mice by the modification of single or multiple genes have been surprisingly unhelpful at resolving this issue. Perhaps these genetic studies only serve to emphasize the multi–organ system nature of diabetes mellitus, in which several defects are required to elicit sufficient dysfunction to overwhelm physiological compensatory mechanisms and produce diabetes.
Nevertheless, investigators have postulated a reasonable series of events to explain the evolution of type 2 diabetes. According to this model, peripheral insulin resistance represents the earliest event, but this is initially compensated by enhanced insulin secretion. Later, the β cell no longer keeps pace with the increased needs, and a relative lack of insulin is followed by an absolute deficiency of the hormone. At about the same time, the liver develops insulin resistance, thus leading to accelerated production of glucose. Whatever the precise sequence of the events by which impaired glucose tolerance matures to diabetes, there is little doubt that insulin resistance represents an important component of the fulminant disease.
Birnbaum MJ. Turning down insulin signaling. Journal of Clinical Investigation. 2001;108(5):655-659.


| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 072-28 | Tumor Necrosis Factor alpha (TNF-alpha), recombinant (Human) | 20 µg | $202 |
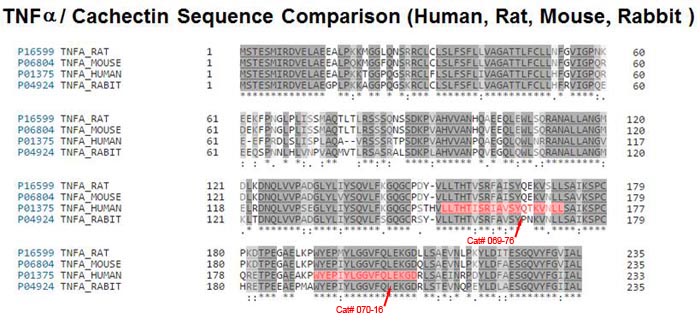
| 069-76 | RMP16 / HAS binding 7-mer-IEGR-TNF-alpha (151-170) (Human) | 100 µg | $356 |
| 054-44 | TRAIL (151-204) (Human) | 200 µg | $444 |
| 054-45 | TRAIL (205-281) (Human) | 200 µg | $508 |
| 054-46 | TRAIL (234-281) (Human) | 200 µg | $444 |
| 054-42 | TRAIL (39-88) (Human) | 200 µg | $444 |
| 054-43 | TRAIL (91-147) amide (Human) | 200 µg | $508 |
| 054-47 | TRAIL-s (39-101) (Human) | 200 µg | $483 |
| 054-80 | TWEAK (117-173) (Human) | 200 µg | $444 |
| 054-79 | TWEAK (174-249) (Human) | 200 µg | $508 |
Social Network Confirmation