

Neuromedin U (NMU) activates two NMU receptors (NMUR1 and NMUR2) and is a useful anti-obesity drug lead. We report discovery of a hexapeptide agonist, 2-thienylacetyl-Trp1-Phe(4-F)2-Arg3-Pro4-Arg5-Asn6-NH2 (4). However, the NMUR1 selectivity and serum stability of this agonist were unsatisfactory. Through a structure-activity relationship study focused on residue 2 of agonist 4, serum stability, and pharmacokinetic properties, we report here the discovery of a novel NMUR1 selective hexapeptide agonist 7b that suppresses body weight gain in mice.
Takayama K, Mori K, Tanaka A, et al. J Med Chem. 2017;60(12):5228-5234.
Group 2 innate lymphoid cells (ILC2s) regulate inflammation, tissue repair and metabolic homeostasis, and are activated by host-derived cytokines and alarmins. Discrete subsets of immune cells integrate nervous system cues, but it remains unclear whether neuron-derived signals control ILC2s. Here we show that neuromedin U (NMU) in mice is a fast and potent regulator of type 2 innate immunity in the context of a functional neuron-ILC2 unit. We found that ILC2s particularly express neuromedin U receptor 1 (Nmur1), and mucosal neurons express NMU. Cell-autonomous activation of ILC2s with NMU resulted in immediate and strong NMUR1-dependent production of innate inflammatory and tissue repair cytokines. NMU controls ILC2s downstream of extracellular signal-regulated kinase and calcium-influx-dependent activation of both calcineurin and nuclear factor of activated T cells (NFAT). NMU treatment in vivo resulted in immediate protective type 2 responses. Accordingly, ILC2-autonomous ablation of Nmur1 led to impaired type 2 responses and poor control of worm infection. Notably, mucosal neurons were found adjacent to ILC2s, and these neurons directly sensed worm products and alarmins to induce NMU and to control innate type 2 cytokines. Our work reveals that neuron-ILC2 cell units confer immediate tissue protection through coordinated neuroimmune sensory responses.
This publication used a NMU peptide (#046-65) from Phoenix Pharmaceuticals for the in vivo and in vitro assays.
Cardoso V, Chesné J, Ribeiro H, et al. Nature. 2017;549(7671):277-281.
The type 2 cytokines interleukin (IL)-4, IL-5, IL-9 and IL-13 have important roles in stimulating innate and adaptive immune responses that are required for resistance to helminth infection, promotion of allergic inflammation, metabolic homeostasis and tissue repair. Group 2 innate lymphoid cells (ILC2s) produce type 2 cytokines, and although advances have been made in understanding the cytokine milieu that promotes ILC2 responses, how ILC2 responses are regulated by other stimuli remains poorly understood. Here we demonstrate that ILC2s in the mouse gastrointestinal tract co-localize with cholinergic neurons that express the neuropeptide neuromedin U (NMU). In contrast to other haematopoietic cells, ILC2s particularly express the NMU receptor 1 (NMUR1). In vitro stimulation of ILC2s with NMU induced rapid cell activation, proliferation, and secretion of the type 2 cytokines IL-5, IL-9 and IL-13 that was dependent on cell-intrinsic expression of NMUR1 and G?q protein. In vivo administration of NMU triggered potent type 2 cytokine responses characterized by ILC2 activation, proliferation and eosinophil recruitment that was associated with accelerated expulsion of the gastrointestinal nematode Nippostrongylus brasiliensis or induction of lung inflammation. Conversely, worm burden was higher in Nmur1-/- mice than in control mice. Furthermore, use of gene-deficient mice and adoptive cell transfer experiments revealed that ILC2s were necessary and sufficient to mount NMU-elicited type 2 cytokine responses. Together, these data indicate that the NMU-NMUR1 neuronal signalling circuit provides a particular mechanism through which the enteric nervous system and innate immune system integrate to promote rapid type 2 cytokine responses that can induce anti-microbial, inflammatory and tissue-protective type 2 responses at mucosal sites.
This publication used a NMU peptide (#046-65) from Phoenix Pharmaceuticals for the in vivo and in vitro assays.
Klose CSN, Mahlakõiv T, Moeller JB, et al. Nature. 2017;549(7671):282-286.
The discovery of neuropeptides provides insights into the regulation of physiological processes. The precursor for the neuropeptide neuromedin U contains multiple consensus sequences for proteolytic processing, suggesting that this precursor might generate additional peptides. We performed immunoaffinity chromatography of rat brain extracts and consequently identified such a product, which we designated neuromedin U precursor-related peptide (NURP). In rat brain, NURP was present as two mature peptides of 33 and 36 residues. Radioimmunoassays revealed NURP immunoreactivity in the pituitary, small intestine, and brain of rats, with the most intense reactivity in the pituitary. Intracerebroventricular administration of NURP to both male and female rats robustly increased plasma concentrations of prolactin but not of other anterior pituitary hormones. In contrast, NURP failed to stimulate prolactin release from dispersed anterior pituitary cells. Pretreatment of rats with bromocriptine, a dopamine receptor agonist, blocked the prolactin-releasing activity of NURP. In rats pretreated with the antagonist sulpiride, intracerebroventricular administration of NURP did not increase plasma prolactin concentrations more than administration of saline. These data suggest that NURP induces prolactin release by acting indirectly on the pituitary; dopamine from the hypothalamus, which inhibits prolactin release, may be involved in this activity of NURP.
Mori K, Ida T, Fudetani M, et al. Sci Rep. 2017;7(1):10468.
Neuromedin U (NMU), a highly conserved peptide in mammals, is implicated in energy homeostasis and glycemic control, and may also be involved in the regulation of adipoinsular axis function. However, the role of NMU in regulating insulin secretion has not been clearly established. In this study, we investigated the role of NMU in the regulation of insulin secretion both in vitro and in vivo. We found that NMU and NMU receptor (NMUR) 1 were expressed in mouse islets and ? cell-derived MIN6-K8 cells. In mice, NMU suppressed glucose-stimulated insulin secretion (GSIS) both in vitro and in vivo. Additionally, an NMUR1 agonist inhibited GSIS in both MIN6-K8 cells and mice islets. Moreover, NMU attenuated intracellular Ca2+ influx in MIN6-K8 cells, potentially causing a decrease in insulin secretion. siNmu-transfected MIN6-K8 cells showed elevated GSIS. Treatment with anti-NMU IgG increased GSIS in isolated mouse pancreatic islets. These results suggested that NMU can act directly on ? cells through NMUR1 in an autocrine or paracrine fashion to suppress insulin secretion. Collectively, our results highlight the crucial role of NMU in suppressing pancreatic insulin secretion, and may improve our understanding of glucose homeostasis.
Zhang W, Sakoda H, Miura A, et al. Biochem Biophys Res Commun. 2017;
Decretins, hormones induced by fasting that suppress insulin production and secretion, have been postulated from classical human metabolic studies. From genetic screens, we identified Drosophila Limostatin (Lst), a peptide hormone that suppresses insulin secretion. Lst is induced by nutrient restriction in gut-associated endocrine cells. limostatin deficiency led to hyperinsulinemia, hypoglycemia, and excess adiposity. A conserved 15-residue polypeptide encoded by limostatin suppressed secretion by insulin-producing cells. Targeted knockdown of CG9918, a Drosophila ortholog of Neuromedin U receptors (NMURs), in insulin-producing cells phenocopied limostatin deficiency and attenuated insulin suppression by purified Lst, suggesting CG9918 encodes an Lst receptor. NMUR1 is expressed in islet ? cells, and purified NMU suppresses insulin secretion from human islets. A human mutant NMU variant that co-segregates with familial early-onset obesity and hyperinsulinemia fails to suppress insulin secretion. We propose Lst as an index member of an ancient hormone class called decretins, which suppress insulin output.
Alfa RW, Park S, Skelly KR, et al. Cell Metab. 2015;21(2):323-33.
Bone remodeling, the function affected in osteoporosis, the most common of bone diseases, comprises two phases: bone formation by matrix-producing osteoblasts and bone resorption by osteoclasts. The demonstration that the anorexigenic hormone leptin inhibits bone formation through a hypothalamic relay suggests that other molecules that affect energy metabolism in the hypothalamus could also modulate bone mass. Neuromedin U (NMU) is an anorexigenic neuropeptide that acts independently of leptin through poorly defined mechanisms. Here we show that Nmu-deficient (Nmu?/?) mice have high bone mass owing to an increase in bone formation; this is more prominent in male mice than female mice. Physiological and cell-based assays indicate that NMU acts in the central nervous system, rather than directly on bone cells, to regulate bone remodeling. Notably, leptin- or sympathetic nervous system–mediated inhibition of bone formation was abolished in Nmu?/? mice, which show an altered bone expression of molecular clock genes (mediators of the inhibition of bone formation by leptin). Moreover, treatment of wild-type mice with a natural agonist for the NMU receptor decreased bone mass. Collectively, these results suggest that NMU may be the first central mediator of leptin-dependent regulation of bone mass identified to date. Given the existence of inhibitors and activators of NMU action, our results may influence the treatment of diseases involving low bone mass, such as osteoporosis.
Sato S, Hanada R, Kimura A, et al. Nat Med. 2007;13(10):1234-40.
Neuromedin U (NMU) is a neuropeptide with potent activity on smooth muscle which was isolated first from porcine spinal cord and later from other species. It is widely distributed in the gut and central nervous system. Peripheral activities of NMU include stimulation of smooth muscle, increase of blood pressure, alteration of ion transport in the gut, control of local blood flow and regulation of adrenocortical function. An NMU receptor has not been molecularly identified. Here we show that the previously described orphan G-protein-coupled receptor FM-3 (ref. 15) and a newly discovered one (FM-4) are cognate receptors for NMU. FM-3, designated NMU1R, is abundantly expressed in peripheral tissues whereas FM-4, designated NMU2R, is expressed in specific regions of the brain. NMU is expressed in the ventromedial hypothalamus in the rat brain, and its level is significantly reduced following fasting. Intracerebroventricular administration of NMU markedly suppresses food intake in rats. These findings provide a molecular basis for the biochemical activities of NMU and may indicate that NMU is involved in the central control of feeding.
This publication used a Neuromedin U peptide, as well as 83 other peptides, from Phoenix Pharmaceuticals.
Howard AD, Wang R, Pong SS, et al. Nature. 2000;406(6791):70-4.
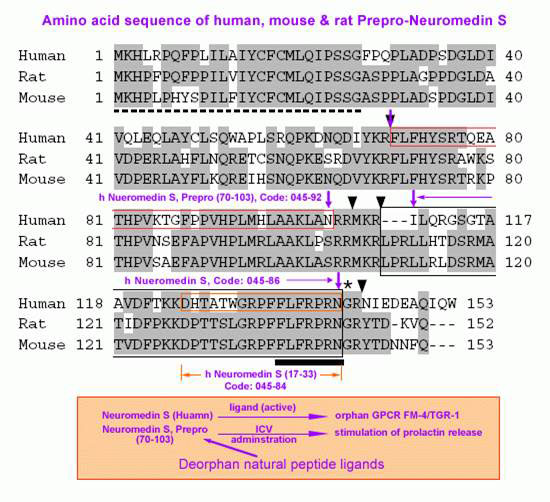
The discovery of neuropeptides has resulted in an increased understanding of novel regulatory mechanisms of certain physiological phenomena. Here we identify a novel neuropeptide of 36 amino-acid residues in rat brain as an endogenous ligand for the orphan G protein-coupled receptor FM-4/TGR-1, which was identified to date as the neuromedin U (NMU) receptor, and designate this peptide ‘neuromedin S (NMS)' because it is specifically expressed in the suprachiasmatic nuclei (SCN) of the hypothalamus. NMS shares a C-terminal core structure with NMU. The NMS precursor contains another novel peptide. NMS mRNA is highly expressed in the central nervous system, spleen and testis. In rat brain, NMS expression is restricted to the core of the SCN and has a diurnal peak under light/dark cycling, but remains stable under constant darkness. Intracerebroventricular administration of NMS in rats activates SCN neurons and induces nonphotic type phase shifts in the circadian rhythm of locomotor activity. These findings suggest that NMS in the SCN is implicated in the regulation of circadian rhythms through autocrine and/or paracrine actions.
Mori K, Miyazato M, Ida T, et al. The EMBO Journal. 2005;24(2):325-335. doi:10.1038/sj.emboj.7600526.

| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 046-65 | Neuromedin U-23 (Mouse) | 100 µg | $242 |
| 046-42 | Neuromedin U-25 (Human) | 200 µg | $203 |
| 046-96 | NMUR1 Selective Agonist 7b | 100 µg | $312 |
| 046-30 | NMUR2 Selective Agonist 6b | 100 µg | $152 |
| 045-96 | NURP33 / prepro-Neuromedin U (104-136) (Human) | 100 µg | $267 |
| 045-97 | NURP33 / prepro-Neuromedin U (106-138) (Rat, Mouse) | 100 µg | $267 |
| 046-76 | NURP36 / prepro-Neuromedin U (104-139) (Human) | 100 µg | $202 |
| H-045-96 | prepro-Neuromedin U (104-136) (Human) - Antibody | 100 µl | $571 |
| B-045-96 | prepro-Neuromedin U (104-136) (Human) - Biotin Labeled | 10 µg | $382 |
| B-G-045-96 | prepro-Neuromedin U (104-136) (Human) - Biotin Labeled Purified IgG | 100 µl | $629 |
Social Network Confirmation