Abstract: Male sexual behavior is innate and rewarding. Despite its centrality to reproduction, a molecularly specified neural circuit governing innate male sexual behavior and reward remains to be characterized. We have discovered a developmentally wired neural circuit necessary and sufficient for male mating.
Bayless DW, Davis C ha O, Yang R, et al. A neural circuit for male sexual behavior and reward. Cell. 2023;186(18):3862-3881.e28.
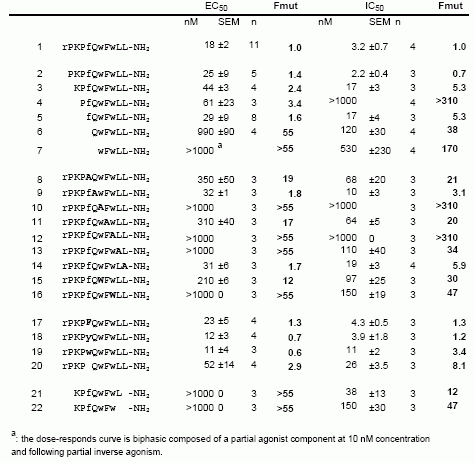
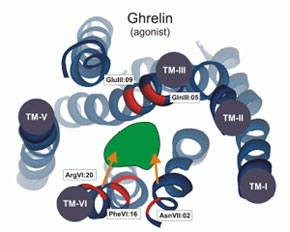
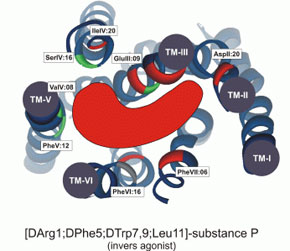
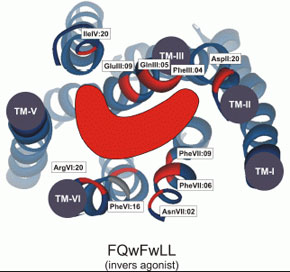
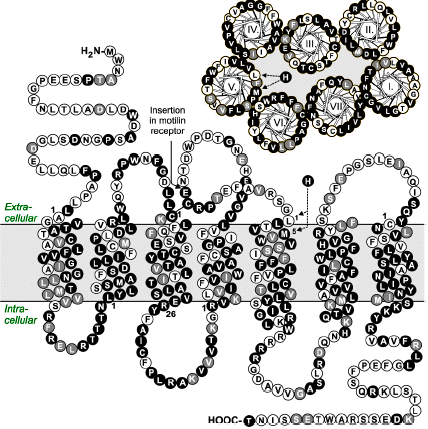
[D-Arg(1),D-Phe(5),D-Trp(7,9),Leu(11)]-substance P functions as a low potency antagonist but a high potency inverse agonist on the ghrelin receptor. Through a systematic deletion and substitution analysis of this peptide the C-terminal, carboxyamidated pentapeptide wFwLX was identified as the core structure, which however in it self displayed relatively low inverse agonist potency. Mutational analysis at 17 selected positions in the main ligand-binding crevice of the ghrelin receptor demonstrated that ghrelin apparently interacts only with residues in the middle part of the pocket i.e. between TM-III, -VI and -VII. In contrast the inverse agonist peptides bind in a pocket, which extends all the way to the extracellular end of TM-II (AspII:20) across between TM-III and TM-VI / -VII over to TM-V and TM-IV. The potency of the main inverse agonist could be improved up to 20-fold by a number of space-generating mutants located relatively deep in the binding pocket at key positions in TM-III, -IV and -V. It is proposed that the inverse agonists prevent the spontaneous receptor activation by inserting relatively deep across the main ligand-binding pocket and sterically blocking the movement of TM-VI and -VII into their inward-bend, active conformation. The combined structure-functional analysis of both the ligand and the receptor allowed for the design of a novel, N-terminally Lys-extended analog of wFwLL, which rescued the high potency, selective inverse agonism being dependent upon both AspII:20 and GluIII:09. The identified pharmacophore can possibly serve as the basis for targeted discovery of also non-peptide inverse agonists for the ghrelin receptor.
Holst B, et al. Mol Pharmacol. 2006 Jun 23; [Epub ahead of print]
Ghrelin is a GH (GH) releasing peptide, which also has an important role as an orexigenic hormone stimulating food intake. By measuring inositol phosphate turnover or by using a reporter assay for transcriptional activity controlled by cAMP responsive elements, the ghrelin receptor showed strong, ligand-independent signaling in transfected COS-7 or HEK293 cells. Ghrelin and a number of the known non-peptide GH secretagogues acted as agonists stimulating inositol phosphate turnover further. In contrast, the low potency ghrelin antagonist, [D-Arg(1),D-Phe(5),D-Trp(7,9),Leu(11)]-substance P was surprisingly found to be a high potency (EC50 = 5.2 nM) full inverse agonist as it decreased the constitutive signaling of the ghrelin receptor down to that observed in un-transfected cells. The homologous motilin receptor functioned as a negative control as it did not display any sign of constitutive activity; however, upon agonist stimulation the motilin receptor signaled as strongly as the un-stimulated ghrelin receptor. It is concluded that the ghrelin receptor is highly constitutively active and that this activity could be of physiological importance in its role as a regulator of both GH secretion and appetite control. It is suggested that inverse agonists for the ghrelin receptor could be particularly interesting for the treatment of obesity.
Holst B, et al. Mol Endocrinol. 2003 Aug 7 [Epub ahead of print].
Ghrelin is a 28-amino-acid peptide, with an essential n-octanoyl modification at Ser3, that elicits growth-hormone (GH) secretion in rats and humans. At present, the mechanisms of ghrelin action and its interactions with other systems controlling GH secretion remain poorly characterized. In this context, the present study was undertaken to obtain information about ontogeny and possible gender differences in the GH-releasing activity of ghrelin, and to delineate its primary site(s) of action at the hypothalamus and/or pituitary. In addition, the interactions between ghrelin and other relevant signals in the control of GH secretion, such as excitatory amino acids (EAAs), nitric oxide (NO) and serotonin, were assessed. Experiments were carried out in infantile-prepubertal animals, when GH pulsatility is not yet established. Systemic administration of ghrelin (25 nmol/rat, i.p.) to 5-, 10- and 23-day-old male and female rats increased plasma GH levels from day 10 onwards. This action was NO dependent, since it disappeared in 23-day-old males after pretreatment with an inhibitor of NO synthase (NAME). Similarly, central infusion of ghrelin (3 nmol/rat, i.c.v.) elicited GH responses in 10- and 23-day-old animals significantly higher than after systemic administration. By contrast, in vitro challenge of pituitary tissue with increasing doses of ghrelin (10(-9)-10(-7) M) failed to enhance GH release into the incubation medium, whereas stimulation with GH-releasing hormone (GHRH; 10(-7) M) or GHRP-6 (10(-7) M) was effective. Finally, effects of ghrelin were blocked by pretreatment with MK-801 and NBQX antagonists of EAA ionotropic receptors and after manipulation of endogenous serotoninergic tone. In addition, the potent releasing activity of EAA agonists NMDA and AMPA was blunted by pretreatment with D-Lys3-GHRP-6, a selective antagonist of the cognate ghrelin receptor, i.e. the GH-secretagogue receptor. In conclusion, our results demonstrate that GH-releasing activity of ghrelin appears early in the infantile period, is NO dependent and involves a primary hypothalamic site of action. The data also demonstrate for the first time the existence of a cross-talk between ghrelin and other neurotransmitter systems, such as EAAs and serotonin, in precise control of GH secretion.
Pinilla L, et al. Neuroendocrinology 2003 Feb;77(2):83-90
BACKGROUND AND AIMS: Ghrelin, an endogenous ligand for growth hormone secretagogue receptor (GHS-R), is an appetite stimulatory signal from the stomach with structural resemblance to motilin. We examined the effects of the gastric peptide ghrelin and GHS-R antagonists on energy balance and glycaemic control in mice.
MATERIALS AND METHODS: Body weight, fat mass, glucose, insulin, and gene expression of leptin, adiponectin, and resistin in white adipose tissue (WAT) were measured after repeated administrations of ghrelin under a high fat diet. Gastric ghrelin gene expression was assessed by northern blot analysis. Energy intake and gastric emptying were measured after administration of GHS-R antagonists. Repeated administration of GHS-R antagonist was continued for six days in ob/ob obese mice.RESULTS: Ghrelin induced remarkable adiposity and worsened glycaemic control under a high fat diet. Pair feeding inhibited this effect. Ghrelin elevated leptin mRNA expression and reduced resistin mRNA expression. Gastric ghrelin mRNA expression during fasting was increased by a high fat diet. GHS-R antagonists decreased energy intake in lean mice, in mice with diet induced obesity, and in ob/ob obese mice; it also reduced the rate of gastric emptying. Repeated administration of GHS-R antagonist decreased body weight gain and improved glycaemic control in ob/ob obese mice.CONCLUSIONS: Ghrelin appears to be closely related to excess weight gain, adiposity, and insulin resistance, particularly under a high fat diet and in the dynamic stage. Gastric peptide ghrelin and GHS-R may be promising therapeutic targets not only for anorexia-cachexia but also for obesity and type 2 diabetes, which are becoming increasingly prevalent worldwide.
Asakawa A, et al. Gut. 2003 Jul;52(7):947-52
.gif)
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| EK-061-05 | Substance P (Human, Rat, Mouse) - EIA Kit | 96 wells | $570 |
| 061-05 | Substance P (Human, Rat, Mouse) | 500 µg | $35 |
| FEK-061-05 | Substance P (Human, Rat, Mouse) - Fluorescent EIA Kit | 96 wells | $624 |
| H-061-05 | Substance P (Human, Rat, Mouse) - Antibody | 50 µl | $225 |
| EK-061-05CE | Substance P (Human, Rat, Mouse) - EIA Kit, CE Mark Certified | 96 wells | $595 |
| RK-061-05 | Substance P (Human, Rat, Mouse) - RIA Kit | 125 tubes | $880 |
| FEK-061-05CE | Substance P (Human, Rat, Mouse) - Fluorescent EIA Kit, CE Mark Certified | 96 wells | $649 |
| 061-40 | CAP-TAC1/ [pGlu6]-Substance P (6-11) / [pGlu1]-Hexa-Substance P | 1mg | $115 |
| 046-57 | Senktide (Selective Neurokinin B Receptor Peptide) / [Asp6, MePhe8]-Suc-Substance P (6-11) | 5 mg | $258 |
| 061-03 | Spantide I / [D-Arg1, D-Trp7, D-Trp9, Leu11]-Substance P | 500 µg | $61 |
Social Network Confirmation