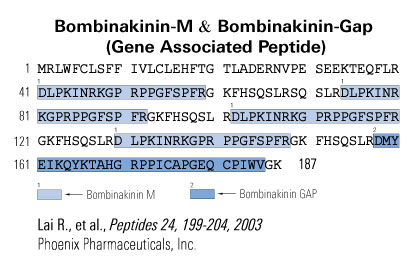
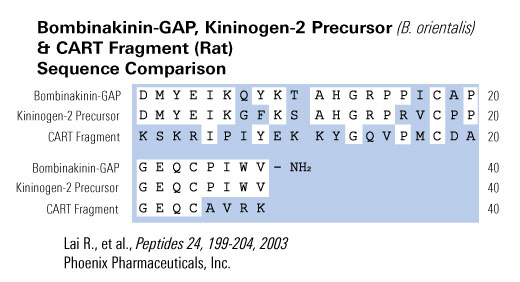
A novel 28-amino acid peptide, termed bombinakinin-GAP, was purified and characterized from skin secretions of the toad Bombina maxima. Its primary structure was established as DMYEIKQYKTAHGRPPICAPGEQCPIWV-NH(2), in which two cysteines form a disulfide bond. A FASTA search of SWISS-PROT databank detected a 32% sequence identity between the sequences of the peptide and a segment of rat cocaine- and amphetamine-regulated transcript (CART). Intracerebroventricular (i.c.v.) administration of the peptide induced a significant decrease in food intake in rats, suggesting that it played a role in the control of feeding by brain. Analysis of its cDNA structure revealed that this peptide is coexpressed with bombinakinin M, a bradykinin-related peptide from the same toad. Bombinakinin-GAP appears to be the first example of a novel class of bioactive peptides from amphibian skin, which may be implicated in feeding behavior.
Lai R, Liu H, Lee WH, Zhang Y. Bombinakinin M gene associated peptide, a novel bioactive peptide from skin secretions of the toad Bombina maxima. Peptides. 2003;24(2):199-204.
Amphibian skin contains rich bradykinin-related peptides, but the mode of biosynthesis of these peptides is unknown. In the present study, a novel bradykinin-related peptide, termed bombinakinin M, was purified from skin secretions of the Chinese red belly toad Bombina maxima. Its primary sequence was established as DLPKINRKGPRPPGFSPFR that comprises bradykinin extended from its N-terminus by a 10-residue segment DLPKINRKGP. The cDNA structure of bombinakinin M was found to contain a coding region of 624 nucleotides. The encoded precursor of bombinakinin M is composed of a signal peptide, an acidic peptide, six 100% identical copies of a 28-amino-acid peptide unit including bombinakinin M plus a spacer peptide. The sequence of bombinakinin M is preceded by a single basic residue (arginine), which represents the site of cleavage for releasing of mature bombinakinin M. This is the first cDNA cloning of bradykinin-related peptides from amphibian skin. The unique cDNA structure encoding bombinakinin M suggests that the generation modes of bradykinin-related peptides in amphibian skin and in mammalian blood system are different.
Lai R, Liu H, Hui lee W, Zhang Y. Biochem Biophys Res Commun. 2001;286(2):259-63.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 009-39 | Bombinakinin M (Bombina maxima) | 100 µg | $202 |
| B-009-39 | Bombinakinin M (Bombina maxima) - Biotin Labeled | 20 µg | $317 |
| FG-009-39A | Bombinakinin M (Bombina maxima) - FAM Labeled | 1 nmol | $287 |
| FR-009-39 | Bombinakinin M (Bombina maxima) - Rhodamine Labeled | 1 nmol | $287 |
| 009-38 | Bombinakinin-GAP | 100 µg | $202 |
| H-009-38 | Bombinakinin-GAP - Antibody | 50 µl | $571 |
| B-009-38 | Bombinakinin-GAP - Biotin Labeled | 20 µg | $317 |
| B-G-009-38 | Bombinakinin-GAP - Biotin Labeled Purified IgG | 100 µl | $635 |
| FG-009-38A | Bombinakinin-GAP - FAM Labeled | 1 nmol | $317 |
| FG-G-009-38A | Bombinakinin-GAP - FAM Labeled Purified IgG | 100 µl | $635 |
Social Network Confirmation