

Micropeptide regulator of β-oxidation (MOXI) is a conserved muscle-enriched protein encoded by an RNA transcript misannotated as non-coding. MOXI localizes to the inner mitochondrial membrane where it associates with the mitochondrial trifunctional protein, an enzyme complex that plays a critical role in fatty acid β-oxidation. Isolated heart and skeletal muscle mitochondria from MOXI knockout mice exhibit a diminished ability to metabolize fatty acids, while transgenic MOXI overexpression leads to enhanced β-oxidation. Additionally, hearts from MOXI knockout mice preferentially oxidize carbohydrates over fatty acids in an isolated perfused heart system compared to wild-type (WT) animals. MOXI knockout mice also exhibit a profound reduction in exercise capacity, highlighting the role of MOXI in metabolic control. The functional characterization of MOXI provides insight into the regulation of mitochondrial metabolism and energy homeostasis and underscores the regulatory potential of additional micropeptides that have yet to be identified.Makarewich CA, Baskin KK, Munir AZ, et al. MOXI Is a Mitochondrial Micropeptide That Enhances Fatty Acid β-Oxidation. Cell Rep. 2018;23(13):3701-3709.
Mitochondria are composed of many small proteins that control protein synthesis, complex assembly, metabolism, and ion and reactive oxygen species (ROS) handling. We show that a skeletal muscle- and heart-enriched long non-coding RNA, LINC00116, encodes a highly conserved 56-amino-acid microprotein that we named mitoregulin (Mtln). Mtln localizes to the inner mitochondrial membrane, where it binds cardiolipin and influences protein complex assembly. In cultured cells, Mtln overexpression increases mitochondrial membrane potential, respiration rates, and Ca2+ retention capacity while decreasing mitochondrial ROS and matrix-free Ca2+. Mtln-knockout mice display perturbations in mitochondrial respiratory (super)complex formation and activity, fatty acid oxidation, tricarboxylic acid (TCA) cycle enzymes, and Ca2+ retention capacity. Blue-native gel electrophoresis revealed that Mtln co-migrates alongside several complexes, including the complex I assembly module, complex V, and supercomplexes. Under denaturing conditions, Mtln remains in high-molecular-weight complexes, supporting its role as a sticky molecular tether that enhances respiratory efficiency by bolstering protein complex assembly and/or stability.
Stein CS, Jadiya P, Zhang X, et al. Cell Rep. 2018;23(13):3710-3720.e8.
BACKGROUND: Trauma causes inflammation by releasing mitochondria (MT) that act as DAMPs. Trauma also increases susceptibility to infection. Human MT contain 13 N-formyl peptides (mtFPs). We studied whether mtFPs released into plasma by clinical injury induce neutrophil (PMN) inflammatory responses, whether their potency reflects their similarity to bacterial FPs and how their presence at clinically relevant concentration affects PMN function.
METHODS: N-terminal sequences of the 13 mtFPs were synthesized. Changes in human PMN cytosolic Ca concentration ([Ca]i) and chemotactic responses to mtFPs were studied. Sequence similarity of mtFPs to the canonical bacterial peptide f-Met-Leu-Phe (fMLF/fMLP) was studied using the BLOSUM62 system. The presence of mtFPs in plasma of trauma patients was assayed by ELISA. The effects of the most potent mtFP (ND6) on PMN signaling and function were then studied at ambient clinical concentrations by serial exposure of native PMN to ND6, chemokines and leukotrienes.RESULTS: Five mtFPs (ND6, ND3, ND4, ND5, and Cox 1) induced [Ca]i flux and chemotaxis in descending order of potency. Evolutionary similarity to fMLF predicted [Ca]i flux and chemotactic potency linearly (R=0.97, R=0.95). Chemoattractant potency was also linearly related to [Ca]i flux induction (R= 0.92). Active mtFPs appear to circulate in significant amounts immediately after trauma and persist through the first week. The most active mtFP, ND6, suppresses responses to physiologic alveolar chemoattractants (CXCL-1, LTB4) as well as to fMLF where CXCL-1 and LTB4 do not suppress FPR-1 responses to mtFPs. Prior FPR-1 inhibition rescues PMN from heterologous suppression of CXCR-1 and BLT-1 by mtFPs.CONCLUSIONS: The data suggest mtFPs released by injured tissue may attract PMN to trauma sites while suppressing PMN responses to other chemoattractants. Inhibition of mtFP-FPR1 interactions might increase PMN recruitment to lung bacterial inoculation after trauma. These findings suggest new paradigms for preventing infections after trauma.LEVEL OF EVIDENCE: Pre-clinical therapeutic study, level II.Kaczmarek E, Hauser CJ, Kwon WY, et al. J Trauma Acute Care Surg. 2018;
Phagocytic neutrophils express formyl peptide receptors (FPRs; FPR1 and FPR2) that distinctly recognize peptides starting with an N-formylated methionine (fMet). This is a hallmark of bacterial metabolism; similar to prokaryotes, the starting amino acid in synthesis of mitochondrial DNA-encoded proteins is an fMet. Mitochondrial cryptic peptides (mitocryptides; MCTs) with an N-terminal fMet could be identified by our innate immune system; however, in contrast to our knowledge about bacterial metabolites, very little is known about the recognition profiles of MCTs. In this study, we determined the neutrophil-recognition profiles and functional output of putative MCTs originating from the N termini of the 13 human mitochondrial DNA-encoded proteins. Six of the thirteen MCTs potently activated neutrophils with distinct FPR-recognition profiles: MCTs from ND3 and ND6 have a receptor preference for FPR1; MCTs from the proteins ND4, ND5, and cytochrome b prefer FPR2; and MCT-COX1 is a dual FPR1/FPR2 agonist. MCTs derived from ND2 and ND4L are very weak neutrophil activators, whereas MCTs from ND1, ATP6, ATP8, COX2, and COX3, do not exert agonistic or antagonistic FPR effects. In addition, the activating MCTs heterologously desensitized IL-8R but primed the response to the platelet-activating factor receptor agonist. More importantly, our data suggest that MCTs have biased signaling properties in favor of activation of the superoxide-generating NADPH oxidase or recruitment of β-arrestin. In summary, we identify several novel FPR-activating peptides with sequences present in the N termini of mitochondrial DNA-encoded proteins, and our data elucidate the molecular basis of neutrophil activation by MCTs.
Gabl M, Sundqvist M, Holdfeldt A, et al. J Immunol. 2018;200(9):3269-3282.
BACKGROUND: Due to common evolutionary origins, mitochondrial DNA (mtDNA) shares many similarities with immunogenic bacterial DNA. MtDNA is recognized as a pro-inflammatory damage-associated molecular pattern (DAMP) with a pathogenic role in several inflammatory diseases. We hypothesised that mtDNA is released during active disease, serving as a key pro-inflammatory factor in inflammatory bowel disease (IBD).METHODS: Between 2014 and 2015, we collected plasma separated within 2 hours of sampling from 97 prospectively recruited IBD patients (67 ulcerative colitis [UC] and 30 Crohn's disease [CD]) and 40 non-IBD controls. We measured circulating mtDNA using quantitative polymerase chain reaction (amplifying mitochondria COXIII/ND2 genes) and also in mouse colitis induced by dextran sulfate-sodium (DSS). We used a mass spectometry approach to detect free plasma mitochondrial formylated peptides. Furthermore, we examined for mitochondrial damage using electron microscopy (EM) and TLR9 expression, the target for mtDNA, in human intestinal IBD mucosa.RESULTS: Plasma mtDNA levels were increased in UC and CD (both P < 0.0001) compared with non-IBD controls. These levels were significantly correlated to blood (C-reactive protein, albumin, white cell count), clinical and endoscopic markers of severity, and disease activity. In active UC, we identified 5 mitochondrial formylated peptides (the most abundant being fMMYALF with known chemoattractant function) in plasma. We observed mitochondrial damage in inflamed UC mucosa and significantly higher fecal MtDNA levels (vs non-IBD controls [P < 0.0001]), which supports gut mucosal mitochondrial DAMP release as the primary source. In parallel, plasma mtDNA levels increased during induction of acute DSS colitis and were associated with more severe colitis (P < 0.05). In active IBD, TLR9+ lamina propria inflammatory cells were significantly higher in UC and CD compared with controls (P < 0.05).CONCLUSIONS: We present the first evidence to show that mtDNA is released during active IBD. MtDNA is a potential mechanistic biomarker, and our data point to mtDNA-TLR9 as a therapeutic target in IBD.Boyapati RK, Dorward DA, Tamborska A, et al. Mitochondrial DNA Is a Pro-Inflammatory Damage-Associated Molecular Pattern Released During Active IBD. Inflamm Bowel Dis. 2018;
The major pathophysiological characteristic of systemic inflammatory response syndrome (SIRS) and sepsis is the loss of control of vascular tone and endothelial barrier dysfunction. These changes are attributed to pro-inflammatory mediators. It has been proposed that in patients and rats without infection, cell components from damaged tissue are the primary instigators of vascular damage. Mitochondria share several characteristics with bacteria, and when fragments of mitochondria are released into the circulation after injury, they are recognized by the innate immune system. N-Formyl peptides are common molecular signatures of bacteria and mitochondria and are known to play a role in the initiation of inflammation by activating the formyl peptide receptor (FPR). We have demonstrated that infusion of mitochondrial N-formyl peptides (F-MIT) leads to sepsis-like symptoms, including vascular leakage. We have also observed that F-MIT, via FPR activation, elicits changes in cytoskeleton-regulating proteins in endothelial cells. Therefore, we hypothesize that these FPR-mediated changes in cytoskeleton can cause endothelial cell contraction and, consequently vascular leakage. Here, we propose that endothelial FPR is a key contributor to impaired barrier function in SIRS and sepsis patients following trauma.
Wenceslau CF, Mccarthy CG, Webb RC. Front Immunol. 2016;7:297.
Previously, we have shown that copy number of the maternally inherited mitochondrial genome is strictly regulated during development. Indeed, oocytes, embryos and stem cells regulate their mtDNA copy number so that, once cells commit to a specific lineage, they increase their mtDNA copy number in a cell-specific manner.1, 2, 3 This enables cells to meet their needs for ATP in order that they can support their specialized functional requirements. For example, neurons have a high requirement for ATP generated through OXPHOS to generate action potentials and mediate neurotransmitter activity. Indeed, in undifferentiated cells, the control of mtDNA copy number is a tightly regulated process as these cells establish the mtDNA set point, which is defined as a small number of mtDNA copies per cell that remains constant until cells initiate differentiation.1, 2 Cells then use this population of mtDNA as a template for replication as differentiation ensues. However, cancer cells fail to undergo these transformations as they are unable to mediate mtDNA replication.We further identified specific changes in mtDNA variants in tumors derived from mtDNA-depleted cells. MtDNA variants differ amongst cancer types, but they were also modulated at different stages of tumor progression in two models with decreases at early progression and increases at late progression in our glioblastoma and osteosarcoma models but an overall reduction in our multiple myeloma model at late progression, suggesting that this is cancer type-dependent. We further discovered that the mtDNA-encoded complex I gene ND6 is a hotspot for mtDNA variants in tumorigenesis. One plausible explanation is that ND6 is the only protein-coding gene on the light strand of mtDNA and the last gene to be replicated, increasing the possibility of proofreading errors.
W T Y Lee and J C St. John, Cell Death Dis. 2016 Mar; 7(3): e2171.
Fifty percent of trauma patients who present sepsis-like syndrome do not have bacterial infections. This condition is known as systemic inflammatory response syndrome (SIRS). A unifying factor of SIRS and sepsis is cardiovascular collapse. Trauma and severe blood loss cause the release of endogenous molecules known as damage-associated molecular patterns. Mitochondrial N-formyl peptides (F-MIT) are damage-associated molecular patterns that share similarities with bacterial N-formylated peptides and are potent immune system activators. The goal of this study was to investigate whether F-MIT trigger SIRS, including hypotension and vascular collapse via formyl peptide receptor (FPR) activation. We evaluated cardiovascular parameters in Wistar rats treated with FPR or histamine receptor antagonists and inhibitors of the nitric oxide pathway before and after F-MIT infusion. F-MIT, but not nonformylated peptides or mitochondrial DNA, induced severe hypotension via FPR activation and nitric oxide and histamine release. Moreover, F-MIT infusion induced hyperthermia, blood clotting, and increased vascular permeability. To evaluate the role of leukocytes in F-MIT-induced hypotension, neutrophil, basophil, or mast cells were depleted. Depletion of basophils, but not neutrophils or mast cells, abolished F-MIT-induced hypotension. Rats that underwent hemorrhagic shock increased plasma levels of mitochondrial formylated proteins associated with lung damage and antagonism of FPR ameliorated hemorrhagic shock-induced lung injury. Finally, F-MIT induced vasodilatation in isolated resistance arteries via FPR activation; however, F-MIT impaired endothelium-dependent relaxation in the presence of blood. These data suggest that F-MIT may be the link among trauma, SIRS, and cardiovascular collapse.
Wenceslau CF, Mccarthy CG, Szasz T, Goulopoulou S, Webb RC. Am J Physiol Heart Circ Physiol. 2015;308(7):H768-77.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 005-08 | MCT-COX1 (Human) | 100 µg | $184 |
| 005-09 | MCT-ND3 (Human) | 200 µg | $160 |
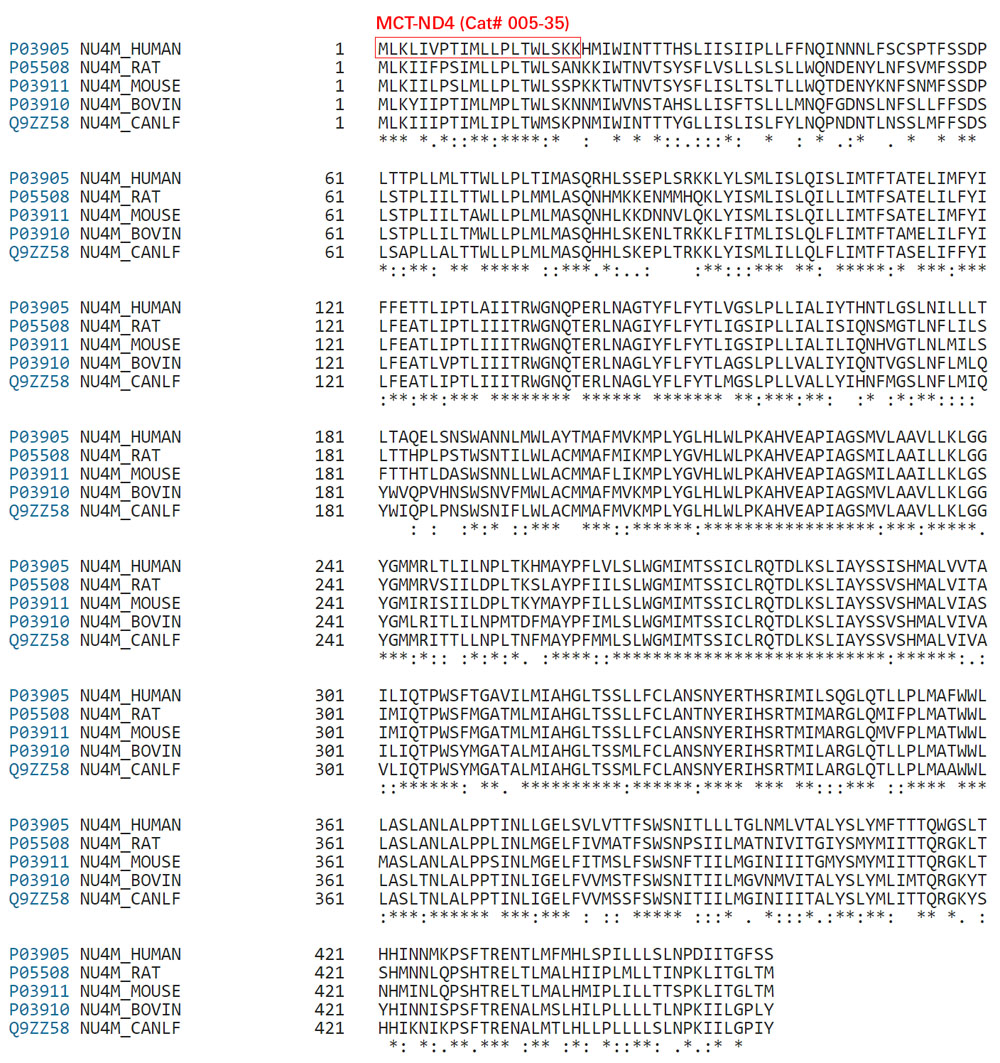
| 005-35 | MCT-ND4 (Human) | 100 µg | $195 |
| 005-48 | MCT-ND6 / F-MIT (Human) | 200 µg | $172 |
| 005-42 | Mitocryptide-1 (MCT-1) (Human) | 100 µg | $230 |
| H-005-42 | Mitocryptide-1 (MCT-1) (Human) - Antibody | 100 µl | $444 |
| B-G-005-42 | Mitocryptide-1 (MCT-1) (Human) - Biotin Labeled Purified IgG | 100 µl | $444 |
| FC3-G-005-42 | Mitocryptide-1 (MCT-1) (Human) - Cy3 Labeled Purified IgG | 100 µl | $794 |
| FC5-G-005-42 | Mitocryptide-1 (MCT-1) (Human) - Cy5 Labeled Purified IgG | 100 µl | $794 |
| EK-005-42 | Mitocryptide-1 (MCT-1) (Human) - EIA Kit | 96 wells | $570 |
Social Network Confirmation