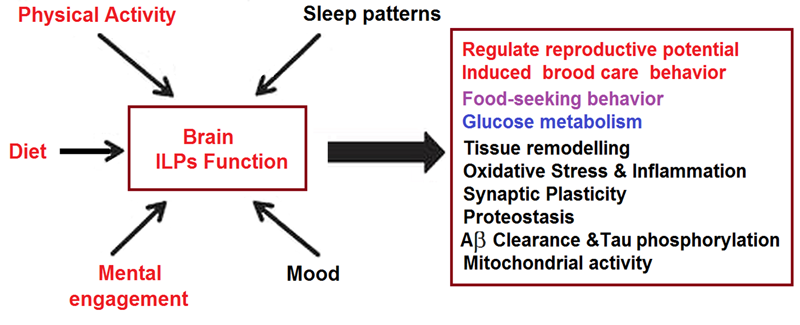
Individual susceptibility to anxiety disorders after maladaptive responses to stress is not well understood. We now report that while exploring stress responses in mice after traumatic brain injury (TBI), a condition associated to stress susceptibility, we observed that the anxiogenic effects of either TBI or exposure to life-threatening experiences (predator) were blocked when both stressors were combined. Because TBI increases the entrance into the brain of serum insulin-like growth factor I (IGF-I), a known modulator of anxiety with a wide range of concentrations in the human population, we then determined whether circulating IGF-I is related to anxiety measures. In mice, anxiety-like responses to predator were inversely related to circulating IGF-I levels. Other indicators of mood regulation such as sensitivity to dexamethasone suppression and expression levels of blood and brain FK506 binding protein 5 (FKBP5), a co-chaperone of the glucocorticoid receptor that regulates its activity, were also associated to circulating IGF-I. Indeed, brain FKBP5 expression in mice was stimulated by IGF-I. In addition, we observed in a large human cohort (n = 2686) a significant relationship between plasma IGF-I and exposure to recent stressful life events, while FKBP5 expression in blood cells was significantly associated to plasma IGF-I levels. Collectively, these data indicate that circulating IGF-I appears to be involved in mood homeostasis across different species. Furthermore, the data in mice allow us to indicate that IGF-I may be acting at least in part by modulating FKBP5 expression.
Santi A, Bot M, Aleman A, Penninx BWJH, Aleman IT. Circulating insulin-like growth factor I modulates mood and is a biomarker of vulnerability to stress: from mouse to man. Transl Psychiatry. 2018;8(1):142.
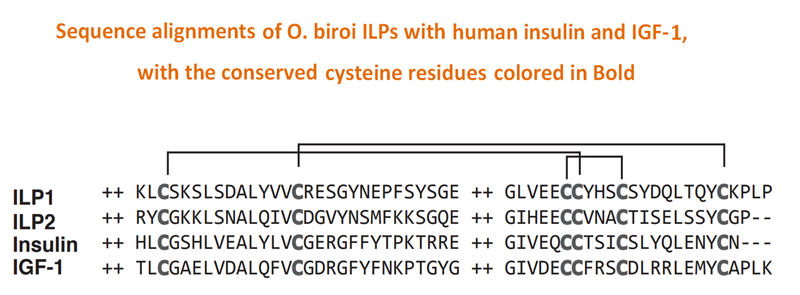
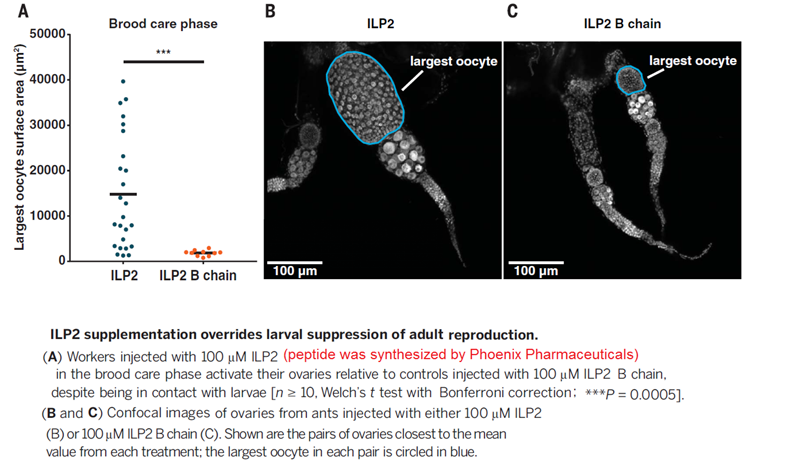
Queens and workers of eusocial Hymenoptera are considered homologous to the reproductive and brood care phases of an ancestral subsocial life cycle. However, the molecular mechanisms underlying the evolution of reproductive division of labor remain obscure. Using a brain transcriptomics screen, we identified a single gene, insulin-like peptide 2 (ilp2), which is always up-regulated in ant reproductives, likely because they are better nourished than their nonreproductive nestmates. In clonal raider ants (Ooceraea biroi), larval signals inhibit adult reproduction by suppressing ilp2, thus producing a colony reproductive cycle reminiscent of ancestral subsociality. However, increasing ILP2 peptide levels overrides larval suppression, thereby breaking the colony cycle and inducing a stable division of labor. These findings suggest a simple model for the origin of ant eusociality via nutritionally determined reproductive asymmetries potentially amplified by larval signals.
Chandra V, Fetter-pruneda I, Oxley PR, et al. Social regulation of insulin signaling and the evolution of eusociality in ants. Science. 2018;361(6400):398-402.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 036-75 | Con-Ins G1 | 100 µg | $562 |
| 036-77 | [Glu4A, 12B, Pro5B]-Con-Ins G1 / sCon-Ins G1 | 100 µg | $436 |
| 036-47 | Harpegnathos-Sal Insulin (31-107) amide | 100 µg | $474 |
| 036-45 | Harpegnathos-Sal Insulin (39-96) amide | 100 µg | $436 |
| 036-93 | ILP-1 (Ooceraea biroi) | 100 µg | $553 |
| 036-91 | ILP-2 (Ooceraea biroi) | 100 µg | $490 |
| 036-49 | LIRP (68-130) / C-peptide of locust insulin-related protein | 100 µg | $436 |
Social Network Confirmation