PURPOSE: Protease nexin-1 (PN-1), a serpin encoded by the SERPINE2 gene, has serine protease inhibitory activity and neurotrophic properties in the brain. PN-1 inhibits retinal angiogenesis; however, PN-1's neurotrophic capacities in the retina have not yet been evaluated. Pigment epithelium-derived factor (PEDF) is a serpin that exhibits neurotrophic and antiangiogenic activities but lacks protease inhibitory properties. The aim of this study is to compare PN-1 and PEDF.
METHODS: Sequence comparisons were performed using computer bioinformatics programs. Mouse and bovine eyes, human retina tissue, and ARPE-19 cells were used to prepare RNA and protein samples. Interphotoreceptor matrix lavage was obtained from bovine eyes. Gene expression and protein levels were evaluated with reverse-transcription PCR (RT-PCR) and western blotting, respectively. Recombinant human PN-1, a version of PN-1 referred to as PN-1[R346A] lacking serine protease inhibitory activity, and PEDF proteins were used, as well as synthetic peptides designed from PEDF and PN-1 sequences. Survival activity in serum-starved, rat-derived retinal precursor (R28) cells was assessed with terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) cell death assays. Bcl2 levels were measured with RT-PCR.
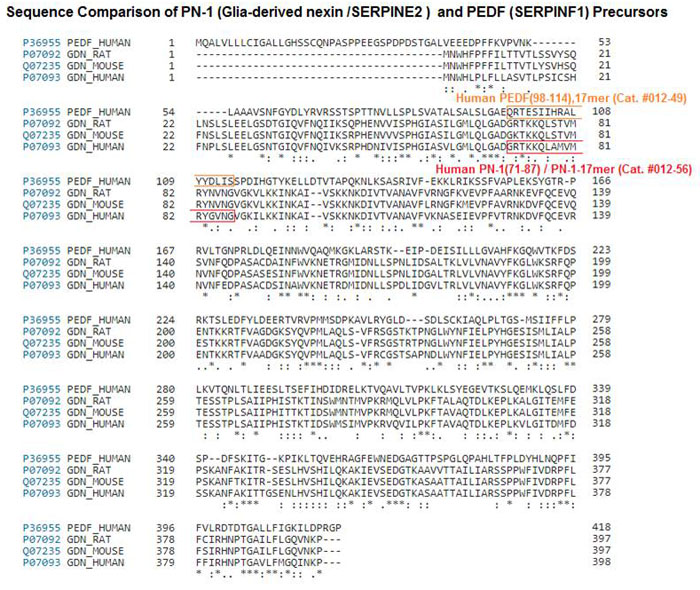
RESULTS: PN-1 is analogous in primary and tertiary structure to PEDF. A region in PN-1 shares homology with the neurotrophic active region of PEDF, a 17-residue region within alpha helix C. The native human retina, ARPE-19 cells, and murine RPE and retina expressed the gene for PN-1 (SERPINE2 and Serpine2 mRNA). The retina, ARPE-19 cell lysates, and bovine interphotoreceptor matrix contained PN-1 protein. The addition of PN-1, PN-1[R346A], or the 17mer peptide of PN-1 to serum-starved retina cells decreased the number of TUNEL-positive nuclei relative to the untreated cells, such as PEDF. PN-1, PN-1[R346A], and PN-1-17mer treatments increased the Bcl2 transcript levels in serum-starved cells, as seen with PEDF.
CONCLUSIONS: PN-1 and PEDF share structural and functional features, and expression patterns in the retina. These serpins' mechanisms of action as cell survival factors are independent of serine protease.
Social Network Confirmation