
Abstract: β-amyloid (Aβ)-dependent neuronal hyperactivity is believed to contribute to the circuit dysfunction that characterizes the early stages of Alzheimer's disease (AD). Although experimental evidence in support of this hypothesis continues to accrue, the underlying pathological mechanisms are not well understood. In this experiment, we used mouse models of Aβ-amyloidosis to show that hyperactivation is initiated by the suppression of glutamate reuptake. Hyperactivity occurred in neurons with preexisting baseline activity, whereas inactive neurons were generally resistant to Aβ-mediated hyperactivation. Aβ-containing AD brain extracts and purified Aβ dimers were able to sustain this vicious cycle. Our findings suggest a cellular mechanism of Aβ-dependent neuronal dysfunction that can be active before plaque formation.Zott B, Simon MM, Hong W, et al. A vicious cycle of β amyloid-dependent neuronal hyperactivation. Science. 2019;365(6453):559-565.
Summary: Aggregation of the β-amyloid peptide (Aβ) in brain regions serving memory and cognition is thought to initiate Alzheimer's disease (AD) (1). Despite myriad studies of the neurobiological effects of the peptide, two central questions remain unsettled: What forms of Aβ are the principal bioactive neurotoxins in humans, and precisely how do these forms undermine neuronal function? Attempts to therapeutically lower or neutralize Aβ in humans have so far failed to definitively slow AD symptoms, and successful approaches may require answers to these two queries. On page 559 of this issue, Zott et al. (2) provide compelling evidence that the first answer is soluble Aβ dimers, and the second answer is through hyperexcitability of glutamatergic neurons when the dimers interfere with the reuptake of extracellular glutamate.Selkoe DJ. Early network dysfunction in Alzheimer's disease. Science. 2019;365(6453):540-541.
Abstract: Cerebral amyloid beta (Aβ) deposits are the main early pathology of Alzheimer's disease (AD). However, abundant Aβ deposits also occur spontaneously in the brains of many healthy people who are free of AD with advancing aging. A crucial unanswered question in AD prevention is why AD does not develop in some elderly people, despite the presence of Aβ deposits. The answer may lie in the composition of Aβ oligomer isoforms in the Aβ deposits of healthy brains, which are different from AD brains. However, which Aβ oligomer triggers the transformation from aging to AD pathogenesis is still under debate. Some researchers insist that the Aβ 12-mer causes AD pathology, while others suggest that the Aβ dimer is the crucial molecule in AD pathology. Aged rhesus monkeys spontaneously develop Aβ deposits in the brain with striking similarities to those of aged humans. Thus, rhesus monkeys are an ideal natural model to study the composition of Aβ oligomer isoforms and their downstream effects on AD pathology. In this study, we found that Aβ deposits in aged monkey brains included 3-mer, 5-mer, 9-mer, 10-mer, and 12-mer oligomers, but not 2-mer oligomers. The Aβ deposits, which were devoid of Aβ dimers, induced glial pathology (microgliosis, abnormal microglia morphology, and astrocytosis), but not the subsequent downstream pathologies of AD, including Tau pathology, neurodegeneration, and synapse loss. Our results indicate that the Aβ dimer plays an important role in AD pathogenesis. Thus, targeting the Aβ dimer is a promising strategy for preventing AD.Zhang J, Chen B, Lu J, et al. Brains of rhesus monkeys display Aβ deposits and glial pathology while lacking Aβ dimers and other Alzheimer's pathologies. Aging Cell. 2019;18(4):e12978.
Abstract: Aggregation of amyloid β42 (Aβ42) is one of the hallmarks of Alzheimer's disease (AD). There are numerous naturally occurring products that suppress the aggregation of Aβ42, but the underlying mechanisms remain to be elucidated. Based on NMR and MS spectroscopic analysis, we propose three structural characteristics found in natural products required for the suppressive activity against Aβ42 aggregation (i.e., oligomerization by targeting specific amino acid residues on this protein). These characteristics include (1) catechol-type flavonoids that can form Michael adducts with the side chains of Lys16 and 28 in monomeric Aβ42 through flavonoid autoxidation; (2) non-catechol-type flavonoids with planarity due to α,β-unsaturated carbonyl groups that can interact with the intermolecular β-sheet region in Aβ42 aggregates, especially aromatic rings such as those of Phe19 and 20; and (3) carboxy acid derivatives with triterpenoid or anthraquinoid that can generate a salt bridge with basic amino acid residues such as Lys16 and 28 in the Aβ42 dimer or trimer. Here, we summarize the recent body of knowledge concerning amyloidogenic inhibitors, particularly in functional food components and Kampo medicine, and discuss their application in the treatment and prevention of AD.Murakami K, Irie K. Three Structural Features of Functional Food Components and Herbal Medicine with Amyloid β42 Anti-Aggregation Properties. Molecules. 2019;24(11)
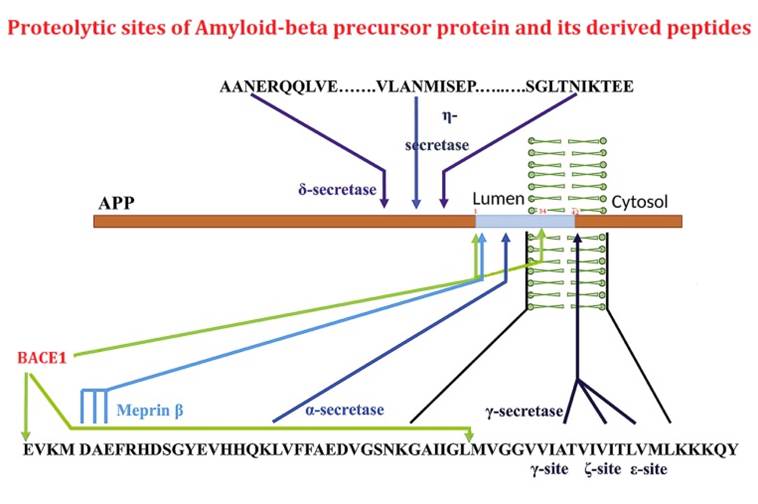
The beta-site APP cleaving enzyme 1 (BACE1) is known primarily for its initial cleavage of the amyloid precursor protein (APP), which ultimately leads to the generation of Aβ peptides. Here, we provide evidence that altered BACE1 levels and activity impact the degradation of Aβ40 and Aβ42 into a common Aβ34 intermediate. Using human cerebrospinal fluid (CSF) samples from the Amsterdam Dementia Cohort, we show that Aβ34 is elevated in individuals with mild cognitive impairment who later progressed to dementia. Furthermore, Aβ34 levels correlate with the overall Aβ clearance rates in amyloid positive individuals. Using CSF samples from the PREVENT-AD cohort (cognitively normal individuals at risk for Alzheimer's disease), we further demonstrate that the Aβ34/Aβ42 ratio, representing Aβ degradation and cortical deposition, associates with pre-clinical markers of neurodegeneration. We propose that Aβ34 represents a marker of amyloid clearance and may be helpful for the characterization of Aβ turnover in clinical samples.Liebsch F, Kulic L, Teunissen C, et al. Aβ34 is a BACE1-derived degradation intermediate associated with amyloid clearance and Alzheimer's disease progression. Nat Commun. 2019;10(1):2240.
There is increasing evidence suggesting that amyloidogenic proteins might form deposits in non-neuronal tissues in neurodegenerative disorders such as Alzheimer's or Parkinson's diseases. However, the detection of these aggregation-prone proteins within the human skin has been controversial. Using immunohistochemistry (IHC) and mass spectrometry tissue imaging (MALDI-MSI), fresh frozen human skin samples were analyzed for the expression and localization of neurodegenerative disease-related proteins. While α-synuclein was detected throughout the epidermal layer of the auricular samples (IHC and MALDI-MSI), tau and Aβ34 were also localized to the epidermal layer (IHC). In addition to Aβ peptides of varying length (e.g., Aβ40, Aβ42, Aβ34), we also were able to detect inflammatory markers within the same sample sets (e.g., thymosin β-4, psoriasin). While previous literature has described α-synuclein in the nucleus of neurons (e.g., Parkinson's disease), our current detection of α-synuclein in the nucleus of skin cells is novel. Imaging of α-synuclein or tau revealed that their presence was similar between the young and old samples in our present study. Future work may reveal differences relevant for diagnosis between these proteins at the molecular level (e.g., age-dependent post-translational modifications). Our novel detection of Aβ34 in human skin suggests that, just like in the brain, it may represent a stable intermediate of the Aβ40 and Aβ42 degradation pathway.Akerman SC, Hossain S, Shobo A, et al. Neurodegenerative Disease-Related Proteins within the Epidermal Layer of the Human Skin. J Alzheimers Dis. 2019;69(2):463-478.
Amyloid-β precursor protein (APP) is central to the pathogenesis of Alzheimer's disease, yet its physiological function remains unresolved. Accumulating evidence suggests that APP has a synaptic function mediated by an unidentified receptor for secreted APP (sAPP). Here we show that the sAPP extension domain directly bound the sushi 1 domain specific to the γ-aminobutyric acid type B receptor subunit 1a (GABABR1a). sAPP-GABABR1a binding suppressed synaptic transmission and enhanced short-term facilitation in mouse hippocampal synapses via inhibition of synaptic vesicle release. A 17-amino acid peptide corresponding to the GABABR1a binding region within APP suppressed in vivo spontaneous neuronal activity in the hippocampus of anesthetized Thy1-GCaMP6s mice. Our findings identify GABABR1a as a synaptic receptor for sAPP and reveal a physiological role for sAPP in regulating GABABR1a function to modulate synaptic transmission.
Rice HC, De malmazet D, Schreurs A, et al. Secreted amyloid-β precursor protein functions as a GABAR1a ligand to modulate synaptic transmission. Science. 2019;363(6423)
The aim of the present study was to establish a cell model of Alzheimer's disease (AD) and investigate the neurotoxic effects of β-amyloid (Aβ) on the cytoskeleton. PC12 cells were cultured and treated with Aβ25?35, and cell survival was analyzed with the MTT assay. Cell apoptosis was visualized using 4',6-diamidino-2-phenylindole staining and the terminal deoxynucleotidyl transferase dUTP nick-end labeling assay. Immunocytochemistry and phalloidin staining were used to label the cytoskeleton of PC12 cells. Aβ25-35 was found to induce PC12 cell apoptosis in a dose-dependent manner (P<0.05). Moreover, Aβ25-35 also caused dose-dependent disintegration of the cytoskeleton (P<0.05). Therefore, the PC12 cell cytoskeleton was found to be sensitive to Aβ25-35 neurotoxicity. The disintegration of the cytoskeleton is likely an important pathological alteration in AD, and Aβ is a key molecule involved in AD pathogenesis.Wang L, Cao J, Shi Z, et al. Experimental study on the neurotoxic effect of β-amyloid on the cytoskeleton of PC12 cells. Int J Mol Med. 2018;41(5):2764-2770.
Upregulation of neprilysin (NEP) to reduce Aβ accumulation in the brain is a promising strategy for the prevention of Alzheimer's disease (AD). This report describes the design and synthesis of a quenched fluorogenic peptide substrate qf-Aβ(12-16)AAC (with the sequence VHHQKAAC), which has a fluorophore, Alexa-350, linked to the side-chain of its C-terminal cysteine and a quencher, Dabcyl, linked to its N-terminus. This peptide emitted strong fluorescence upon cleavage. Our results showed that qf-Aβ(12-16)AAC is more sensitive to NEP than the previously reported peptide substrates, so that concentrations of NEP as low as 0.03 nM could be detected at peptide concentration of 2 μM. Moreover, qf-Aβ(12-16)AAC had superior enzymatic specificity for both NEP and angiotensin-converting enzyme (ACE), but was inert with other Aβ-degrading enzymes. This peptide, used in conjunction with a previously reported peptide substrate qf-Aβ(1-7)C [which is sensitive to NEP and insulin-degrading enzyme (IDE)], could be used for high-throughput screening of compounds that only upregulate NEP. The experimental results of cell-based activity assays using both qf-Aβ(1-7)C and qf-Aβ(12-16)AAC as the substrates confirm that somatostatin treatment most likely upregulates IDE, but not NEP, in neuroblastoma cells.
Chen PT, Chen CL, Lin LT, et al. PLoS ONE. 2016;11(4):e0153360.
Epidemiological studies show that patients with type 2 diabetes (T2DM) and individuals with a diabetes-independent elevation in blood glucose have an increased risk for developing dementia, specifically dementia due to Alzheimer's disease (AD). These observations suggest that abnormal glucose metabolism likely plays a role in some aspects of AD pathogenesis, leading us to investigate the link between aberrant glucose metabolism, T2DM, and AD in murine models. Here, we combined two techniques – glucose clamps and in vivo microdialysis – as a means to dynamically modulate blood glucose levels in awake, freely moving mice while measuring real-time changes in amyloid-β (Aβ), glucose, and lactate within the hippocampal interstitial fluid (ISF). In a murine model of AD, induction of acute hyperglycemia in young animals increased ISF Aβ production and ISF lactate, which serves as a marker of neuronal activity. These effects were exacerbated in aged AD mice with marked Aβ plaque pathology. Inward rectifying, ATP-sensitive potassium (K(ATP)) channels mediated the response to elevated glucose levels, as pharmacological manipulation of K(ATP) channels in the hippocampus altered both ISF Aβ levels and neuronal activity. Taken together, these results suggest that K(ATP) channel activation mediates the response of hippocampal neurons to hyperglycemia by coupling metabolism with neuronal activity and ISF Aβ levels.
Macauley SL, Stanley M, Caesar EE, et al. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J Clin Invest. 2015;125(6):2463-7.
The β-amyloid precursor protein undergoes cleavages by β- and γ-secretasses yielding amyloid-β peptides (Aβ) that accumulate in Alzheimer's disease. Subsequently, Aβ peptides are targets of additional truncations or endoproteolytic cleavages explaining the diversity of Aβ-related fragments recovered in cell media or pathologic human fluids. Here, we focused on Aβ1-34 (Aβ34) that has been detected both in vitro and in vivo and that derives from the hydrolysis of Aβ by β-secretase. We have obtained and fully characterized by immunologic and biochemical approaches, a polyclonal antibody that specifically recognizes the C-terminus of Aβx-34. We present immunohistochemical evidence for the presence of Aβx-34 in the brain of 3xTg mice and Alzheimer's disease-affected human brains. Finally, we demonstrate a neprilysin-mediated degradation process of Aβ34 and the ability of synthetic Aβ34 to protect HEK cells overexpressing either wild type or Swedish-mutated β-amyloid precursor protein from apoptosis.Caillava C, Ranaldi S, Lauritzen I, et al. Study on Aβ34 biology and detection in transgenic mice brains. Neurobiol Aging. 2014;35(7):1570-81.
Extracellular and intraneuronal accumulation of amyloid-beta (Aβ) peptide aggregates in the brain has been hypothesized to play an important role in the neuropathology of Alzheimer’s Disease (AD). The main Aβ variants detected in the human brain are Aβ1-40 and Aβ1-42, however a significant proportion of AD brain Aβ consists also of N-terminal truncated species. Pyroglutamate-modified Aβ peptides have been demonstrated to be the predominant components among all N-terminal truncated Aβ species in AD brains and represent highly desirable and abundant therapeutic targets. The current review describes the properties and localization of two pyroglutamate-modified Aβ peptides, AβN3(pE) and AβN11(pE), in the brain. The role of glutaminyl cyclase (QC) in the formation of these peptides is also addressed. In addition, two potential therapeutic strategies, the inhibition of QC and immunotherapy approaches, and clinical trials aimed to target these important pathological Aβ species are reviewed.
Perez-Garmendia R, Gevorkian G. Pyroglutamate-Modified Amyloid Beta Peptides: Emerging Targets for Alzheimer´s Disease Immunotherapy. Current Neuropharmacology. 2013;11(5):491-498. doi:10.2174/1570159X11311050004 .
BACKGROUND: The order and magnitude of pathologic processes in Alzheimer's disease are not well understood, partly because the disease develops over many years. Autosomal dominant Alzheimer's disease has a predictable age at onset and provides an opportunity to determine the sequence and magnitude of pathologic changes that culminate in symptomatic disease.
METHODS: In this prospective, longitudinal study, we analyzed data from 128 participants who underwent baseline clinical and cognitive assessments, brain imaging, and cerebrospinal fluid (CSF) and blood tests. We used the participant's age at baseline assessment and the parent's age at the onset of symptoms of Alzheimer's disease to calculate the estimated years from expected symptom onset (age of the participant minus parent's age at symptom onset). We conducted cross-sectional analyses of baseline data in relation to estimated years from expected symptom onset in order to determine the relative order and magnitude of pathophysiological changes.
RESULTS: Concentrations of amyloid-beta (A?)42 in the CSF appeared to decline 25 years before expected symptom onset. A? deposition, as measured by positron-emission tomography with the use of Pittsburgh compound B, was detected 15 years before expected symptom onset. Increased concentrations of tau protein in the CSF and an increase in brain atrophy were detected 15 years before expected symptom onset. Cerebral hypometabolism and impaired episodic memory were observed 10 years before expected symptom onset. Global cognitive impairment, as measured by the Mini–Mental State Examination and the Clinical Dementia Rating scale, was detected 5 years before expected symptom onset, and patients met diagnostic criteria for dementia at an average of 3 years after expected symptom onset.
CONCLUSIONS: We found that autosomal dominant Alzheimer's disease was associated with a series of pathophysiological changes over decades in CSF biochemical markers of Alzheimer's disease, brain amyloid deposition, and brain metabolism as well as progressive cognitive impairment. Our results require confirmation with the use of longitudinal data and may not apply to patients with sporadic Alzheimer's disease.
Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795-804.
Full-length amyloid beta peptides (Aβ1–40/42) form neuritic amyloid plaques in Alzheimer’s disease (AD) patients and are implicated in AD pathology. However, recent transgenic animal models cast doubt on their direct role in AD pathology. Nonamyloidogenic truncated amyloid-beta fragments (Aβ11–42 and Aβ17–42) are also found in amyloid plaques of AD and in the preamyloid lesions of Down syndrome, a model system for early-onset AD study. Very little is known about the structure and activity of these smaller peptides, although they could be the primary AD and Down syndrome pathological agents. Using complementary techniques of molecular dynamics simulations, atomic force microscopy, channel conductance measurements, calcium imaging, neuritic degeneration, and cell death assays, we show that nonamyloidogenic Aβ9–42 and Aβ17–42 peptides form ion channels with loosely attached subunits and elicit single-channel conductances. The subunits appear mobile, suggesting insertion of small oligomers, followed by dynamic channel assembly and dissociation. These channels allow calcium uptake in amyloid precursor protein-deficient cells. The channel mediated calcium uptake induces neurite degeneration in human cortical neurons. Channel conductance, calcium uptake, and neurite degeneration are inhibited by zinc, a blocker of amyloid ion channel activity. Thus, truncated A? fragments could account for undefined roles played by full length A?s and provide a unique mechanism of AD and Down syndrome pathologies. The toxicity of nonamyloidogenic peptides via an ion channel mechanism necessitates a reevaluation of the current therapeutic approaches targeting the nonamyloidogenic pathway as avenue for AD treatment.
Jang H, Arce FT, Ramachandran S, et al. Truncated beta-amyloid peptide channels provide an alternative mechanism for Alzheimer's Disease and Down syndrome. Proc Natl Acad Sci USA. 2010;107(14):6538-43.
CONTEXT: Blood-based analytes may be indicators of pathological processes in Alzheimer disease (AD). OBJECTIVE: To identify plasma proteins associated with AD pathology using a combined proteomic and neuroimaging approach.
DESIGN: Discovery-phase proteomics to identify plasma proteins associated with correlates of AD pathology. Confirmation and validation using immunodetection in a replication set and an animal model.
SETTING: A multicenter European study (AddNeuroMed) and the Baltimore Longitudinal Study of Aging.
PARTICIPANTS: Patients with AD, subjects with mild cognitive impairment, and healthy controls with standardized clinical assessments and structural neuroimaging.
MAIN OUTCOME MEASURES: Association of plasma proteins with brain atrophy, disease severity, and rate of clinical progression. Extension studies in humans and transgenic mice tested the association between plasma proteins and brain amyloid. RESULTS: Clusterin/apolipoprotein J was associated with atrophy of the entorhinal cortex, baseline disease severity, and rapid clinical progression in AD. Increased plasma concentration of clusterin was predictive of greater fibrillar amyloid-beta burden in the medial temporal lobe. Subjects with AD had increased clusterin messenger RNA in blood, but there was no effect of single-nucleotide polymorphisms in the gene encoding clusterin with gene or protein expression. APP/PS1 transgenic mice showed increased plasma clusterin, age-dependent increase in brain clusterin, as well as amyloid and clusterin colocalization in plaques. CONCLUSIONS: These results demonstrate an important role of clusterin in the pathogenesis of AD and suggest that alterations in amyloid chaperone proteins may be a biologically relevant peripheral signature of AD.
Thambisetty M, Simmons A, Velayudhan L, et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010;67(7):739-48.
Alzheimer's disease constitutes a rising threat to public health. Despite extensive research in cellular and animal models, identifying the pathogenic agent present in the human brain and showing that it confers key features of Alzheimer's disease has not been achieved. We extracted soluble amyloid-beta protein (Abeta) oligomers directly from the cerebral cortex of subjects with Alzheimer's disease. The oligomers potently inhibited long-term potentiation (LTP), enhanced long-term depression (LTD) and reduced dendritic spine density in normal rodent hippocampus. Soluble Abeta from Alzheimer's disease brain also disrupted the memory of a learned behavior in normal rats. These various effects were specifically attributable to Abeta dimers. Mechanistically, metabotropic glutamate receptors were required for the LTD enhancement, and N-methyl D-aspartate receptors were required for the spine loss. Co-administering antibodies to the Abeta N-terminus prevented the LTP and LTD deficits, whereas antibodies to the midregion or C-terminus were less effective. Insoluble amyloid plaque cores from Alzheimer's disease cortex did not impair LTP unless they were first solubilized to release Abeta dimers, suggesting that plaque cores are largely inactive but sequester Abeta dimers that are synaptotoxic. We conclude that soluble Abeta oligomers extracted from Alzheimer's disease brains potently impair synapse structure and function and that dimers are the smallest synaptotoxic species.
(a) TBS extracts of AD (top panel) and control (bottom panel) brains were subjected to non-denaturing SEC. SEC fractions were lyophilized and WB’d with 2G3+21F12; molecular weights (in kDa) on left. Note that Aß monomer and dimer in the AD TBS extract is recovered from material that elutes at the end of the void volume (fractions 3/4). Control (Con) brain extracts were devoid of Aß. (b) Summary LTP data (means ± SEMs) for slices treated with SEC fractions from AD or Con TBS extracts (n=6 slices for all samples), as characterized in Fig. 3a. (c) Representative WB (2G3+21F12) of IP-SEC fractionation of AD TBS and Con TBS. TBS extracts (500 µL) were immunoprecipitated with 3D6 (3 µg/mL), eluted with sample buffer and subjected to SEC. Dimer-enriched (fractions 7–8) and monomer-enriched (fractions 10–11) IP-SEC fractions were separately pooled, as were corresponding fractions from Con TBS. (d) Summary LTP data (means ± SEMs) for slices treated with IP-SEC fractions of AD and control brain TBS extracts, as characterized in Fig. 3c (Con fractions 10–11, n=5; AD fractions 10–11, n=5; Con fractions 7–8, n=7; AD fractions 7–8, n=7). (e) Mutant Aß40-S26C forms dimers under oxidizing conditions (ox), which can be reduced to monomers by treating with ß-ME (red). Silver stain was performed with 100 ng wildtype Aß40 (wt) peptide or the mutant peptide (f) Summary LTP data (means ± SEMs) for slices treated with 5, 50, or 100 nM of either wt Aß40 (black) or oxidized Aß40-S26C (red) reveals that the oxidized Aß40-S26C dimer inhibits LTP with much greater potency (100 nM Aß40-S26C, n=4; n=5 for all other treatments). The vehicle controls (plotted at 0 nM) were 50 mM ammonium acetate (n=4) for the S26C peptide and 0.1% ammonium hydroxide (n=4) for wt peptide. Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837-42.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| G-018-71 | Amyloid beta 40-S26C dimer / [A-beta S26C]2 (Human) - Purified IgG Antibody | 200 µg | $571 |
| 018-07 | Amyloid-beta Protein (1-42) (Human) | 200 µg | $212 |
| FC5-018-01 | Amyloid-beta Protein (1-40) (Human) - Cy5 Labeled | 1 nmol | $794 |
| 018-71 | Amyloid beta 40-S26C dimer/ [A-beta 40 S26C]2 (Human) | 100 µg | $382 |
| T-018-07 | Amyloid-beta Protein (1-42) (Human) - I-125 Labeled | 10 µCi | $1082 |
| 018-74 | APP 17mer peptide / APP770 (204-220) (Human) | 500 µg | $128 |
| RK-018-73 | Amyloid beta 40-S26C monomer (Human) - RIA Kit | 125 tubes | $880 |
| 018-73 | Amyloid beta 40-S26C monomer / A-beta 40 S26C monomer (Human) | 100 µg | $317 |
| 018-92 | Amyloid beta-42 S26C dimer/ [A-beta 42 S26C]2 (Human) | 100 ug | $360 |
| 018-91 | Amyloid beta-42 S26C monomer (Human) | 100 ug | $360 |
Social Network Confirmation