The role of cold inducible RNA-binding protein (CIRP) in mediating ischemic brain injury in neonatal rats under chronic hypobaric hypoxia was investigated. The neonatal rat model of chronic hypobaric hypoxia and the cell culture model of SH-SY5Y cells exposed to hypoxia (1% O2) were constructed. The expression of CIRP and hypoxia-inducible factor-1α (HIF-1α) was detected after hypoxic exposure, and the apoptosis-related proteins were analyzed via terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) and western blot analysis to detect neuronal apoptosis. Moreover, the effects of CIRP overexpression on HIF-1α and neuronal apoptosis were identified. Chronic hypobaric hypoxia can lead to HIF-1α expression and neuronal apoptosis in the body. CIRP was induced at early exposure (3 d/7 d). However, the CIRP level in the hypoxic group was obviously lower than that in the control group with the prolongation of exposure time (21 d). In addition, the knockdown of HIF-1α significantly reduced the neuronal apoptosis under hypoxic conditions, indicating that HIF-1α may promote apoptosis during exposure. The overexpression of CIRP significantly inhibited the upregulation of HIF-1α during hypoxia and the HIF-1α-mediated neuronal apoptosis. Results of the current study showed that, CIRP is involved in the ischemic brain injury induced by chronic hypoxia through downregulation of HIF-1α expression.
Chen L, Tian Q, Wang W. Association between CIRP expression and hypoxic-ischemic brain injury in neonatal rats. Exp Ther Med. 2019;18(3):1515-1520.
BACKGROUND: Alcohol abuse affects the brain regions responsible for memory, coordination and emotional processing. Binge alcohol drinking has shown reductions in brain activity, but the molecular targets have not been completely elucidated. We hypothesized that brain cells respond to excessive alcohol by releasing a novel inflammatory mediator, called cold inducible RNA-binding protein (CIRP), which is critical for the decreased brain metabolic activity and impaired cognition.METHODS: Male wild type (WT) mice and mice deficient in CIRP (CIRP-/-) were studied before and after exposure to binge alcohol level by assessment of relative brain glucose metabolism with fluorodeoxyglucose (18FDG) and positron emission tomography (PET). Mice were also examined for object-place memory (OPM) and open field (OF) tasks.RESULTS: Statistical Parametric Analysis (SPM) of 18FDG-PET uptake revealed marked decreases in relative glucose metabolism in distinct brain regions of WT mice after binge alcohol. Regional analysis (post hoc) revealed that while activity in the temporal (secondary visual) and limbic (entorhinal/perirhinal) cortices was decreased in WT mice, relative glucose metabolic activity was less suppressed in the CIRP-/- mice. Group and condition interaction analysis revealed differing responses in relative glucose metabolism (decrease in WT mice but increase in CIRP-/- mice) after alcohol in brain regions including the hippocampus and the cortical amygdala where the percent changes in metabolic activity correlated with changes in object discrimination performance. Behaviorally, alcohol-treated WT mice were impaired in exploring a repositioned object in the OPM task, and were more anxious in the OF task, whereas CIRP-/- mice were not impaired in these tasks.CONCLUSION: CIRP released from brain cells could be responsible for regional brain metabolic hypoactivity leading to cognitive impairment under binge alcohol conditions.Jacob A, Ma Y, Nasiri E, et al. Extracellular cold inducible RNA-binding protein mediates binge alcohol-induced brain hypoactivity and impaired cognition in mice. Mol Med. 2019;25(1):24.
Extracellular cold-inducible RNA-binding protein (CIRP) exaggerates inflammation and tissue injury in sepsis. Neutrophil extracellular traps (NETs) are released by activated neutrophils during sepsis. NETs contribute to pathogen clearance, but excessive NET formation (NETosis) causes inflammation and tissue damage. Peptidylarginine deiminase 4 (PAD4) is associated with NETosis by increasing histone citrullination and chromatin decondensation. We hypothesized that CIRP induces NETosis in the lungs during sepsis via upregulating PAD4 expression. Sepsis was induced in C57BL/6 wild-type (WT) and CIRP-/- mice by cecal ligation and puncture (CLP). After 20 h of CLP induction, NETs in the lungs of WT and CIRP-/- mice were quantified by flow cytometry by staining the single cell suspensions with MPO and CitH3 Abs. PAD4 expression in the lungs of WT and CIRP-/- mice after sepsis was assessed by Western blotting. In vitro effects of recombinant mouse (rm) CIRP for NETosis and PAD4 expression in the bone marrow-derived neutrophils (BMDN) were assessed by flow cytometry and Western blotting, respectively. After 20 h of CLP, NETosis in the lungs was significantly decreased in CIRP-/- mice compared to WT mice, which also correlated with the decreased PAD4 expression. Intratracheal administration of rmCIRP into WT mice significantly increased NETosis and PAD4 expression in the lungs compared to vehicle-injected mice. In vitro culture of BMDN with rmCIRP significantly increased NETosis and PAD4 expression compared to PBS-treated control. Fluorescence microscopy revealed typical web-like structures consistent with NETs in rmCIRP-treated BMDN. Thus, CIRP serves as a novel inducer of NETosis via PAD4 during sepsis.Ode Y, Aziz M, Jin H, Arif A, Nicastro JG, Wang P. Cold-inducible RNA-binding Protein Induces Neutrophil Extracellular Traps in the Lungs during Sepsis. Sci Rep. 2019;9(1):6252.
INTRODUCTION: Neonatal sepsis remains a leading cause of infant mortality. Cold-inducible RNA binding protein (CIRP) is an inflammatory mediator that induces TNF-α production in macrophages. C23 is a CIRP-derived peptide that blocks CIRP from binding its receptor. We therefore hypothesized that treatment with C23 reduces systemic inflammation and protects the lungs in neonatal sepsis.METHODS: Sepsis was induced in C56BL/6 mouse pups (5-7?days) by intraperitoneal injection of adult cecal slurry (0.525?mg/g body weight, LD100). One hour later pups received retroorbital injection of C23 (8?mg/kg) or vehicle (normal saline). Ten hours after sepsis induction, blood and tissues were collected for analysis.RESULTS: C23 treatment resulted in a 58% and 69% reduction in serum levels of proinflammatory cytokines IL-6 and IL-1β, respectively, and a 40% and 45% reduction of AST and LDH, as compared to vehicle-treated septic pups. In the lungs, C23 treatment reduced expression of cytokines IL-6 and IL-1β by 78% and 74%. In addition, the mRNA level of neutrophil chemoattractants KC and MIP-2 was reduced by 84% and 74%, respectively. These results corresponded to a reduction in histologic lung injury score. Vehicle-treated pups scored 0.49?±?0.19, while C23 treatment reduced scores to 0.29?±?0.12 (p?CONCLUSIONS: Inhibition of CIRP with C23 treatment is protective in septic neonatal mice as demonstrated by reduced inflammatory markers systemically and in the lung. Therefore, C23 has promising therapeutic potential in treatment of neonatal sepsis.Denning NL, Yang WL, Hansen L, Prince J, Wang P. C23, an oligopeptide derived from cold-inducible RNA-binding protein, suppresses inflammation and reduces lung injury in neonatal sepsis. J Pediatr Surg. 2019;
Cold-inducible RNA-binding protein (CIRP) is a novel sepsis inflammatory mediator and C23 is a putative CIRP competitive inhibitor. Therefore, we hypothesized that C23 can ameliorate sepsis-associated injury to the lungs and kidneys. First, we confirmed that C23 dose-dependently inhibited TNF-α release, IκBα degradation, and NF-κB nuclear translocation in macrophages stimulated with CIRP. Next, we observed that male C57BL/6 mice treated with C23 (8 mg/kg BW) at 2 h after cecal ligation and puncture (CLP) had lower serum levels of LDH, ALT, IL-6, TNF-α, and IL-1β (reduced by ≥39%) at 20 h after CLP compared with mice treated with vehicle. C23-treated mice also had improved lung histology, less TUNEL-positive cells, lower serum levels of creatinine (34%) and BUN (26%), and lower kidney expression of NGAL (50%) and KIM-1 (86%). C23-treated mice also had reduced lung and kidney levels of IL-6, TNF-α, and IL-1β. E-selectin and ICAM-1 mRNA was significantly lower in C23-treated mice. The 10-day survival after CLP of vehicle-treated mice was 55%, while that of C23-treated mice was 85%. In summary, C23 decreased systemic, lung, and kidney injury and inflammation, and improved the survival rate after CLP, suggesting that it may be developed as a new treatment for sepsis.
Zhang F, Brenner M, Yang WL, Wang P. A cold-inducible RNA-binding protein (CIRP)-derived peptide attenuates inflammation and organ injury in septic mice. Sci Rep. 2018;8(1):3052.
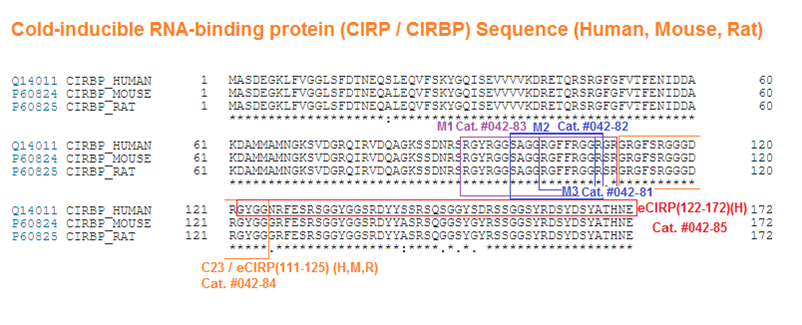
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 042-81 | M3 / eCIRP (101-107) (Human, Mouse, Rat) | 100ug | $122 |
| 042-82 | M2 / eCIRP (97-108) (Human, Mouse, Rat) | 100ug | $181 |
| 042-83 | M1 / eCIRP (91-110) (Human) | 100ug | $240 |
| 042-84 | C23 / eCIRP (101-107) (Human, Mouse, Rat) | 100ug | $252 |
| 042-85 | eCIRP (122-172) (Human) | 100ug | $385 |
| 042-86 | eCIRP (92-118) (Human) | 100ug | $300 |
Social Network Confirmation