GEP44 (Cat. # 028-97), GEP12 (Cat. # 028-98) are made according the reference and Figure from:
Sci Rep. 2023 Jun 12;13(1):9554. doi: 10.1038/s41598-023-36178-1

Abstract: The N-methyl-D-aspartate (NMDA) receptor is a glutamate-activated cation channel that is critical to many processes in the brain. Genome-wide association studies suggest that glutamatergic neurotransmission and NMDA receptor-mediated synaptic plasticity are important for body weight homeostasis1. Here we report the engineering and preclinical development of a bimodal molecule that integrates NMDA receptor antagonism with glucagon-like peptide-1 (GLP-1) receptor agonism to effectively reverse obesity, hyperglycaemia and dyslipidaemia in rodent models of metabolic disease. GLP-1-directed delivery of the NMDA receptor antagonist MK-801 affects neuroplasticity in the hypothalamus and brainstem. Importantly, targeting of MK-801 to GLP-1 receptor-expressing brain regions circumvents adverse physiological and behavioural effects associated with MK-801 monotherapy. In summary, our approach demonstrates the feasibility of using peptide-mediated targeting to achieve cell-specific ionotropic receptor modulation and highlights the therapeutic potential of unimolecular mixed GLP-1 receptor agonism and NMDA receptor antagonism for safe and effective obesity treatment.
Petersen J, Ludwig MQ, Juozaityte V, et al. GLP-1-directed NMDA receptor antagonism for obesity treatment. Nature. 2024;629(8014):1133-1141.
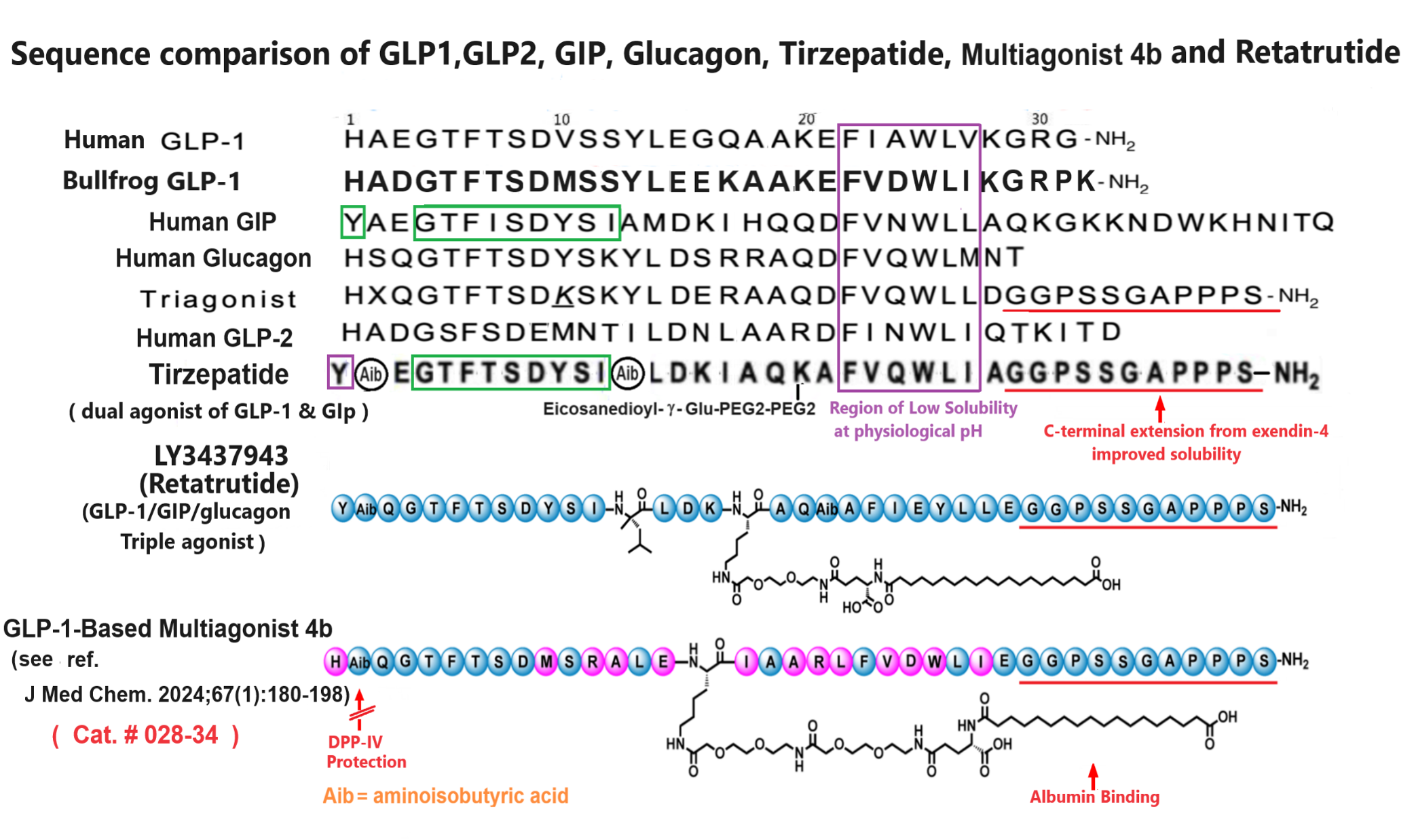
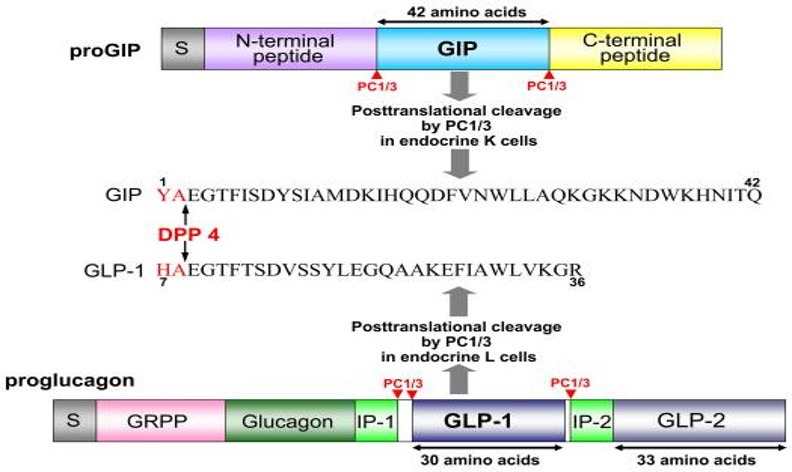
Abstract: In this study, we aimed to discover novel GLP-1 analogues from natural sources. We investigated GLP-1 analogues from fish and amphibians, and bullfrog GLP-1 (bGLP-1) showed the highest potency. Starting with bGLP-1, we explored the structure-activity relationship and performed optimization and long-acting modifications, resulting in a potent analogue called 2f. Notably, 2f exhibited superior effects on food intake, glycemic control, and body weight compared to semaglutide. Furthermore, we explored the usefulness of bGLP-1 in designing GLP-1-based multiagonists. Using the bGLP-1 sequence, we designed novel dual GLP-1/glucagon receptor agonists and triple GLP-1/GIP/glucagon receptor agonists. The selected dual GLP-1/glucagon receptor agonist 3o and triple GLP-1/GIP/glucagon receptor agonist 4b exhibited significant therapeutic effects on lipid regulation, glycemic control, and body weight. Overall, our study highlights the potential of discovering potent GLP-1 receptor agonists from natural sources. Additionally, utilizing natural GLP-1 analogues for designing multiagonists presents a practical approach for developing antiobesity and antidiabetic agents.
Jiang N, Su D, Chen D, et al. Discovery of a novel glucagon-like peptide-1 (GLP-1) analogue from bullfrog and investigation of its potential for designing glp-1-based multiagonists. J Med Chem. 2024;67(1):180-198.
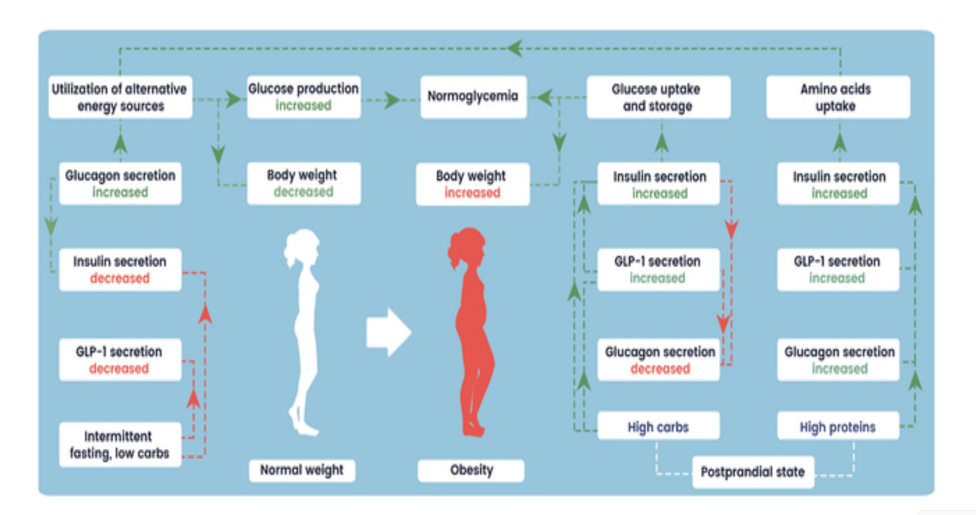
Abstract: The increasing prevalence of "diabesity," a combination of type 2 diabetes and obesity, poses a significant global health challenge. Unhealthy lifestyle factors, including poor diet, sedentary behavior, and high stress levels, combined with genetic and epigenetic factors, contribute to the diabesity epidemic. Diabesity leads to various significant complications such as cardiovascular diseases, stroke, and certain cancers. Incretin-based therapies, such as GLP-1 receptor agonists and dual hormone therapies, have shown promising results in improving glycemic control and inducing weight loss. However, these therapies also come with certain disadvantages, including withdrawal effects. This review aims to provide insights into the cross-interactions of insulin, glucagon, and GLP-1, revealing the complex hormonal dynamics during fasting and postprandial states, impacting glucose homeostasis, energy expenditure, and other metabolic functions. Understanding these hormonal interactions may offer novel hypotheses in the development of "anti-diabesity" treatment strategies. The article also explores the question of the antagonism of insulin and glucagon, providing insights into the potential synergy and hormonal overlaps between these hormones.
Kistkins S, Moser O, et al. From classical dualistic antagonism to hormone synergy: potential of overlapping action of glucagon, insulin and GLP-1 for the treatment of diabesity. Endocrine Connections. 2024;1(aop).
Abstract: Obesity is the fifth leading risk factor for global deaths with numbers continuing to increase worldwide. In the last 20 years, the emergence of pharmacological treatments for obesity based on gastrointestinal hormones has transformed the therapeutic landscape. The successful development of glucagon-like peptide-1 (GLP-1) receptor agonists, followed by the synergistic combined effect of glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonists achieved remarkable weight loss and glycemic control in those with the diseases of obesity and type 2 diabetes. The multiple cardiometabolic benefits include improving glycemic control, lipid profiles, blood pressure, inflammation, and hepatic steatosis. The 2023 phase 2 double-blind, randomized controlled trial evaluating a GLP-1/GIP/glucagon receptor triagonist (retatrutide) in patients with the disease of obesity reported 24.2% weight loss at 48 weeks with 12 mg retatrutide. This review evaluates the current available evidence for GLP-1 receptor agonists, dual GLP-1/GIP receptor co-agonists with a focus on GLP-1/GIP/glucagon receptor triagonists and discusses the potential future benefits and research directions.
Jakubowska A, Roux CW le, Viljoen A. The road towards triple agonists: glucagon-like peptide 1, glucose-dependent insulinotropic polypeptide and glucagon receptor - an update. Endocrinol Metab. 2024;39(1):12-22.
Abstract: The discovery of long-acting incretin receptor agonists represents a major stride forward in tackling the dual epidemic of obesity and diabetes. Here we outline the evolution of incretin-based pharmacotherapy, from exendin-4 to the discovery of the multi-incretin hormone receptor agonists that look set to be our next step toward curing diabetes and obesity. We discuss the multiagonists currently in clinical trials and the improvement in efficacy each new generation of these drugs bring. The success of these agents in preclinical models and clinical trials suggests a promising future for multiagonists in the treatment of metabolic diseases, with the most recent glucose-dependent insulinotropic peptide receptor:glucagon-like peptide 1 receptor:glucagon receptor (GIPR:GLP-1R:GCGR) triagonists rivaling the efficacy of bariatric surgery. However, further research is needed to fully understand how these therapies exert their effect on body weight and in the last section we cover open questions about the potential mechanisms of multiagonist drugs, and the understanding of how gut-brain communication can be leveraged to achieve sustained body weight loss without adverse effects.
Gutgesell RM, Nogueiras R, et al. Dual and triple incretin-based co-agonists: novel therapeutics for obesity and diabetes. Diabetes Ther. Published online April 4, 2024.
Abstract: Within recent decades glucagon receptor (GcgR) agonism has drawn attention as a therapeutic tool for the treatment of type 2 diabetes and obesity. In both mice and humans, glucagon administration enhances energy expenditure and suppresses food intake suggesting a promising metabolic utility. Therefore synthetic optimization of glucagon-based pharmacology to further resolve the physiological and cellular underpinnings mediating these effects has advanced. Chemical modifications to the glucagon sequence have allowed for greater peptide solubility, stability, circulating half-life, and understanding of the structure-function potential behind partial and "super"-agonists. The knowledge gained from such modifications has provided a basis for the development of long-acting glucagon analogues, chimeric unimolecular dual- and tri-agonists, and novel strategies for nuclear hormone targeting into glucagon receptor-expressing tissues. In this review, we summarize the developments leading toward the current advanced state of glucagon-based pharmacology, while highlighting the associated biological and therapeutic effects in the context of diabetes and obesity.
Novikoff A, Muller TD. The molecular pharmacology of glucagon agonists in diabetes and obesity. Peptides. 2023;165:171003.
Abstract: Metabolic effects of glucagon-like peptide 1 (GLP-1) receptor agonists are confounded by weight loss and not fully recapitulated by increasing endogenous GLP-1. We tested the hypothesis that GLP-1 receptor (GLP-1R) agonists exert weight loss-independent, GLP-1R-dependent effects that differ from effects of increasing endogenous GLP-1. Individuals with obesity and prediabetes were randomized to receive for 14 weeks the GLP-1R agonist liraglutide, a hypocaloric diet, or the dipeptidyl peptidase 4 (DPP-4) inhibitor sitagliptin. The GLP-1R antagonist exendin(9-39) and placebo were administered in a two-by-two crossover study during mixed-meal tests. Liraglutide and diet, but not sitagliptin, caused weight loss. Liraglutide improved insulin sensitivity measured by HOMA for insulin resistance (HOMA-IR), the updated HOMA model (HOMA2), and the Matsuda index after 2 weeks, prior to weight loss. Liraglutide decreased fasting and postprandial glucose levels, and decreased insulin, C-peptide, and fasting glucagon levels. In contrast, diet-induced weight loss improved insulin sensitivity by HOMA-IR and HOMA2, but not the Matsuda index, and did not decrease glucose levels. Sitagliptin increased endogenous GLP-1 and GIP values without altering insulin sensitivity or fasting glucose levels, but decreased postprandial glucose and glucagon levels. Notably, sitagliptin increased GIP without altering weight. Acute GLP-1R antagonism increased glucose levels in all groups, increased the Matsuda index and fasting glucagon level during liraglutide treatment, and increased endogenous GLP-1 values during liraglutide and sitagliptin treatments. Thus, liraglutide exerts rapid, weight loss-independent, GLP-1R-dependent effects on insulin sensitivity that are not achieved by increasing endogenous GLP-1.
Mashayekhi M, Nian H, Mayfield D, et al. Weight loss–independent effect of liraglutide on insulin sensitivity in individuals with obesity and prediabetes. Diabetes. 2024;73(1):38-50.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 029-10 | GLP-1 analogue, C-terminal penicillamine-conjugated | 100 µg | $290 |
| 028-34 | GLP-1-Based Multiagonist 4b | 100 µg | $495 |
| 028-99 | GLP-1-MK-801 | 100 µg | $300 |
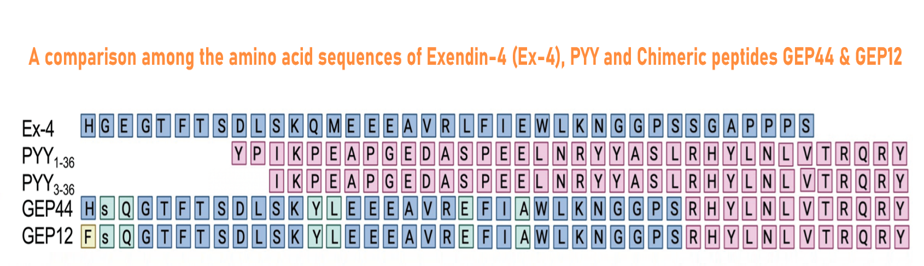
| 028-98 | GLP-1-PYY Chimera GEP12 | 100 μg | $395 |
| 028-97 | GLP-1-PYY Chimera GEP44 | 100 μg | $395 |
| 017-34 | Retatrutide (acetate salt) / LY3437943 | 500 µg | $300 |
Social Network Confirmation