
OBJECTIVE: Glucagon-like peptide-1 (GLP-1) (7-36) amide is a glucoregulatory hormone with insulinotropic and insulinomimetic actions. We determined whether the insulinomimetic effects of GLP-1 are mediated through its principal metabolite, GLP-1 (9-36) amide (GLP-1m). METHODS AND PROCEDURES: Glucose turnover during two, 2-h, euglycemic clamps was measured in 12 lean and 12 obese (BMI <25 or >30 kg/m(2)) male and female subject volunteers with normal oral glucose tolerance test. Saline or GLP-1m were infused from 0 to 60 min in each study. Additionally, seven lean and six obese subjects underwent a third clamp in which the GLP-1 receptor (GLP-1R) antagonist, exendin (9-39) amide was infused from -60 to 60 min with GLP-1m from 0 to 60 min. RESULTS: No glucose infusion was required in lean subjects to sustain euglycemia (glucose clamp) during saline or GLP-1m infusions. However, in obese subjects glucose infusion was necessary during GLP-1m infusion alone in order to compensate for a marked (>50%) inhibition of hepatic glucose production (HGP). Plasma insulin levels remained constant in lean subjects but rose significantly in obese subjects after termination of the peptide infusions. During GLP-1R blockade, infusion of glucose was immediately required upon starting GLP-1m infusions in all subjects due to a more dramatic reduction in HGP, as well as a delayed and modest insulinotropic response. DISCUSSION: We conclude that GLP-1m potently inhibits HGP and is a weak insulinotropic agent. These properties are particularly apparent and pronounced in obese but only become apparent in lean subjects during GLP-1 (7-36) receptor blockade. These previously unrecognized antidiabetogenic actions of GLP-1m may have therapeutic usefulness.
Elahi D, Egan JM, Shannon RP, et al. GLP-1 (9-36) amide, cleavage product of GLP-1 (7-36) amide, is a glucoregulatory peptide. Obesity (Silver Spring). 2008;16(7):1501-9
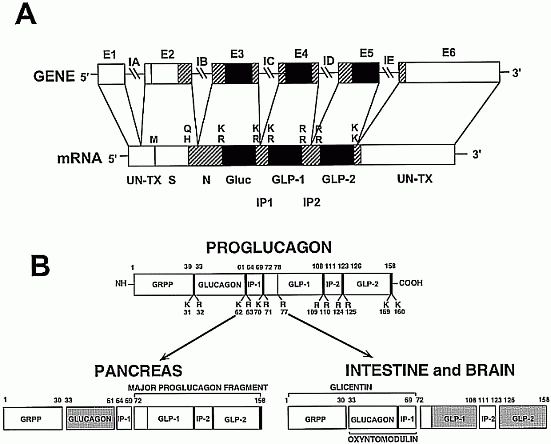
Peptide hormones are secreted from endocrine cells and neurons and exert their actions through activation of G protein-coupled receptors to regulate a diverse number of physiological systems including control of energy homeostasis, gastrointestinal motility, neuroendocrine circuits, and hormone secretion. The glucagon-like peptides, GLP-1 and GLP-2 are prototype peptide hormones released from gut endocrine cells in response to nutrient ingestion that regulate not only energy absorption and disposal, but also cell proliferation and survival. GLP-1 expands islet mass by stimulating pancreatic beta-cell proliferation and induction of islet neogenesis. GLP-1 also promotes cell differentiation, from exocrine cells or immature islet progenitors, toward a more differentiated beta-cell phenotype. GLP-2 stimulates cell proliferation in the gastrointestinal mucosa, leading to expansion of the normal mucosal epithelium, or attenuation of intestinal injury in experimental models of intestinal disease. Both GLP-1 and GLP-2 exert antiapoptotic actions in vivo, resulting in preservation of beta-cell mass and gut epithelium, respectively. Furthermore, GLP-1 and GLP-2 promote direct resistance to apoptosis in cells expressing GLP-1 or GLP-2 receptors. Moreover, an increasing number of structurally related peptide hormones and neuropeptides exert cytoprotective effects through G protein-coupled receptor activation in diverse cell types. Hence, peptide hormones, as exemplified by GLP-1 and GLP-2, may prove to be useful adjunctive tools for enhancement of cell differentiation, tissue regeneration, and cytoprotection for the treatment of human disease.
Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17(2):161-71
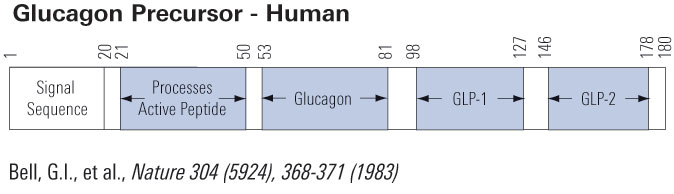
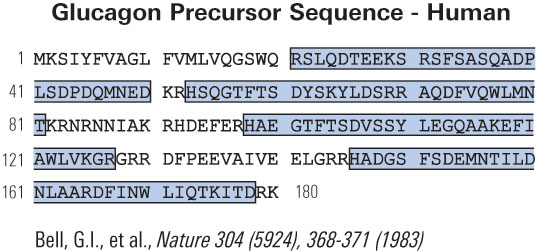
Modern societies have moved from famine to feast and obesity and its co-morbidities now sweep the world as a global epidemic. Numerous scientific laboratories and pharmaceutical companies have taken the challenge and are now exploiting novel molecular targets for treatment of obesity. The pre-proglucagon system constitutes interesting candidates as potential targets for new anti-obesity drugs. In the periphery, pre-proglucagon derived peptides, Glucagon-Like Peptide-1 (GLP-1), Glucagon-Like Peptide-2 (GLP-2) and oxyntomodulin (OXM) are involved in a wide variety of physiological functions, including glucose homeostasis, gastric emptying, intestinal growth, insulin secretion as well as the regulation of food intake. Peripheral administration of GLP-1 derivatives and analogues to both rodents and man have shown promising effects on food intake and body weight suggesting that such therapies constitute potential anti-obesity treatment. In the central nervous system, pre-proglucagon and hence GLP-1, GLP-2 and OXM are exclusively found in a small population of nerve cells in the nucleus of the solitary tract. These constitute a neural pathway linking the "viscero-sensory" brainstem to hypothalamic nuclei involved in energy homeostasis. Intracerebroventricular administration of all of the three derived peptides robustly decrease food intake. It is evident that central GLP-1 agonism probably in combination with GLP-2 and/or OXM agonism constitute a potential pharmacological tool to reduce food intake and maybe also enhance energy expenditure. This and other aspects of the current state of the role of central pre-proglucagon in energy homeostasis are reviewed.
Larsen PJ, Vrang N, Tang-christensen M. Central pre-proglucagon derived peptides: opportunities for treatment of obesity. Curr Pharm Des. 2003;9(17):1373-82
Oxyntomodulin is derived from proglucagon processing in the intestine and the central nervous system. To date, no role in the central nervous system has been demonstrated. We report here that oxyntomodulin inhibits refeeding when injected intracerebroventricularly and into the hypothalamic paraventricular nucleus of 24-h fasted rats [intracerebroventricularly and into the paraventricular nucleus, 1 h, oxyntomodulin (1 nmol), 3.1 +/- 0.5 g; saline, 6.2 +/- 0.4 g; P < 0.005]. In addition, oxyntomodulin inhibits food intake in nonfasted rats injected at the onset of the dark phase (intracerebroventricularly, 1 h: oxyntomodulin, 3 nmol, 1.1 +/- 0.19 g vs. saline, 2.3 +/- 0.2 g; P < 0.05). This effect of oxyntomodulin on feeding is of a similar time course and magnitude as that of an equimolar dose of glucagon-like peptide-1. Other proglucagon-derived products investigated [glucagon, glicentin (intracerebroventricularly, 3 nmol; into the paraventricular nucleus, 1 nmol), and spacer peptide-1 (intracerebroventricularly and into the paraventricular nucleus, 3 nmol)] had no effect on feeding at any time point examined. The anorectic effect of oxyntomodulin (intracerebroventricularly, 3 nmol; into the paraventricular nucleus, 1 nmol) was blocked when it was coadministered with the glucagon-like peptide-1 receptor antagonist, exendin-(9-39) (intracerebroventricularly, 100 nmol; into the paraventricular nucleus, 10 nmol). However, oxyntomodulin has a lower affinity for the glucagon-like peptide-1 receptor compared with glucagon-like peptide-1 (IC(50): oxyntomodulin, 8.2 nM; glucagon-like peptide-1, 0.16 nM). One explanation for this is that there might be an oxyntomodulin receptor to which exendin-(9-39) can also bind and act as an antagonist.
Dakin CL, Gunn I, Small CJ, et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142(10):4244-50
The dorsomedial hypothalamic nucleus harbors leptin sensitive neurons and is intrinsically connected to hypothalamic nuclei involved in feeding behavior. However, it also receives ascending input from the visceroceptive neurons of the brainstem. We have identified a unique glucagon-like-peptide-2 containing neuronal pathway connecting the nucleus of the solitary tract with the dorsomedial hypothalamic nucleus. A glucagon-like-peptide-2 fiber plexus targets neurons expressing its receptor within the dorsomedial hypothalamic nucleus. Pharmacological and behavioral studies confirmed that glucagon-like-peptide-2 signaling is a specific transmitter inhibiting rodent feeding behavior and with potential long-term effects on body weight homeostasis. The glucagon-like-peptide-1 receptor antagonist, Exendin (9-39) is also a functional antagonist of centrally applied glucagon-like-peptide-2.
Tang-christensen M, Larsen PJ, Thulesen J, Rømer J, Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med. 2000;6(7):802-7
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| EK-028-11 | GLP-1 (7-36) amide (Human, Rat, Mouse, Porcine, Bovine, Canine, Ovine) - EIA Kit | 96 wells | $537 |
| 028-11 | GLP-1 (7-36) amide (Human, Rat, Mouse, Porcine, Bovine, Canine, Ovine) | 200 µg | $178 |
| G-028-14 | [Arg34]-GLP-2 (Human) - Purified IgG Antibody | 400 µg | $414 |
| 028-13 | GLP-1 (7-37) (Human, Rat, Mouse, Porcine, Bovine, Canine, Ovine) | 200 µg | $178 |
| FEK-028-11 | GLP-1 (7-36) amide (Human, Rat, Mouse, Porcine, Bovine, Canine, Ovine) - Fluorescent EIA Kit | 96 wells | $537 |
| 028-09 | GLP-1 (Human, Rat, Mouse, Porcine, Bovine, Canine, Ovine) | 200 µg | $97 |
| EK-028-14 | [Arg34]-GLP-2 (Human) - EIA Kit | 96 wells | $537 |
| 028-12 | GLP-1 (9-36) amide (Human, Rat, Mouse, Porcine, Bovine, Canine, Ovine) | 200 µg | $189 |
| H-028-11 | GLP-1 (7-36) amide (Human, Rat, Mouse, Porcine, Bovine, Canine, Ovine) - Antibody | 50 µl | $414 |
| EK-028-11CE | GLP-1 (7-36) amide (Human, Rat, Mouse, Porcine, Bovine, Canine, Ovine) - EIA Kit, CE Mark Certified | 96 wells | $565 |
Social Network Confirmation