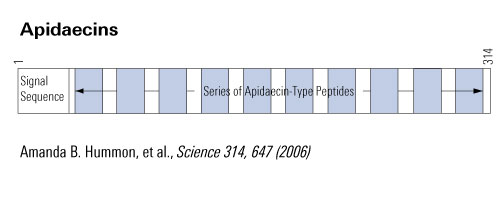
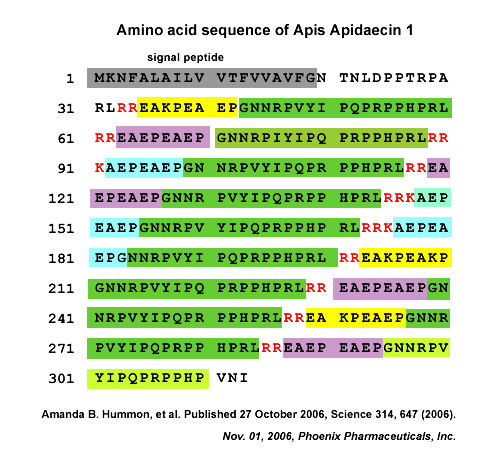
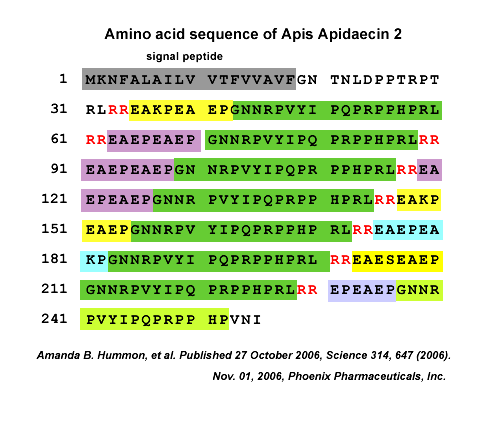
Apidaecins (apidaecin-type peptides) refer to a series of small, proline-rich (Pro-rich), 18- to 20-residue peptides produced by insects. They are the largest group of Pro-rich antimicrobial peptides (AMPs) known to date. Structurally, apidaecins consist of two regions, the conserved (constant) region, responsible for the general antibacterial capacity, and the variable region, responsible for the antibacterial spectrum. The small, gene-encoded and unmodified apidaecins are predominantly active against many gram-negative bacteria by special antibacterial mechanisms. The mechanism of action by which apidaecins kill bacteria involves an initial non-specific binding of the peptides to an outer membrane (OM) component. This binding is followed by invasion of the periplasmic space, and by a specific and essentially irreversible combination with a receptor/docking molecule that may be a component of a permease-type transporter system on inner membrane (IM). In the final step, the peptide is translocated into the interior of the cell where it meets its ultimate target. Evidence that apidaecins are non-toxic for human and animal cells is a prerequisite for using them as novel antibiotic drugs. This review presents the biodiversity, structure-function relationships, and mechanism of action of apidaecins.
Li WF, Ma GX, Zhou XX. Apidaecin-type peptides: biodiversity, structure-function relationships and mode of action. Peptides. 2006;27(9):2350-9.
Pro-rich antimicrobial peptides are a group of linear peptides of innate immunity isolated from mammals and invertebrates, and characterised by a high content of proline residues (up to 50%). Members of this group are predominantly active against Gram-negative bacterial species which they kill by a non-lytic mechanism, at variance with the majority of the known antimicrobial peptides. Evidence is accumulating that the Pro-rich peptides enter the cells without membrane lysis and, once in the cytoplasm, bind to, and inhibit the activity of specific molecular targets essential to bacterial growth, thereby causing cell death. This mode of action makes these peptides suitable for drug development efforts. In addition to antibacterial action, PR-39, one of the better characterised Pro-rich peptides from mammals, exerts other potentially exploitable biological activities, such as induction of syndecan expression in mesenchymal cells and inhibition of the NADPH oxidase activity of neutrophils, suggesting a role of this peptide in wound repair and inflammation. PR-39 also exerts a protective effect in various animal models of ischemia-reperfusion injury, preventing the post-ischemic oxidant production, and is a potent inducer of angiogenesis both in vitro and in vivo. Although the physiological relevance of all these effects has not yet been established, the above observations underscore the therapeutic potential of this peptide in a number of complex processes such as inflammation, wound repair, ischemia-reperfusion injury, and angiogenesis.
Gennaro R, Zanetti M, Benincasa M, Podda E, Miani M. Pro-rich antimicrobial peptides from animals: structure, biological functions and mechanism of action. Curr Pharm Des. 2002;8(9):763-78.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 078-09 | Apidaecin 1 & 2 Gene Peptide, (Asn-13-Leu) | 500 µg | $167 |
| 078-04 | Apidaecin 1 & 2 Gene Peptide, (Pro-10-Leu) | 500 µg | $133 |
| 078-08 | Apidaecin 1 & 2 Gene Peptide, (Pro-10-Pro) | 500 µg | $133 |
| 078-06 | Apidaecin 1 & 2 Gene Peptide, (Pro-8-Leu) | 500 µg | $133 |
| 078-07 | Apidaecin 1 & 2 Gene Peptide, (Pro-8-Pro) | 500 µg | $133 |
| 078-05 | Apidaecin 1 & 2 Gene Peptide, (Pro-9-Arg) | 500 µg | $133 |
| 078-11 | Apidaecin 1 & 2 Gene Peptide, [Glu3]-(Glu-8-Pro) | 500 µg | $133 |
| 078-14 | Apidaecin 1 & 2 Gene Peptide, [Ile6]-(Gly-16-Pro) | 200 µg | $167 |
| 078-01 | Apidaecin 1 & 2 Gene Peptide, [Ile6]-(Gly-18-Leu) | 200 µg | $167 |
| 078-10 | Apidaecin 1 & 2 Gene Peptide, [Lys3]-(Glu-8-Pro) | 500 µg | $133 |
Social Network Confirmation