 |
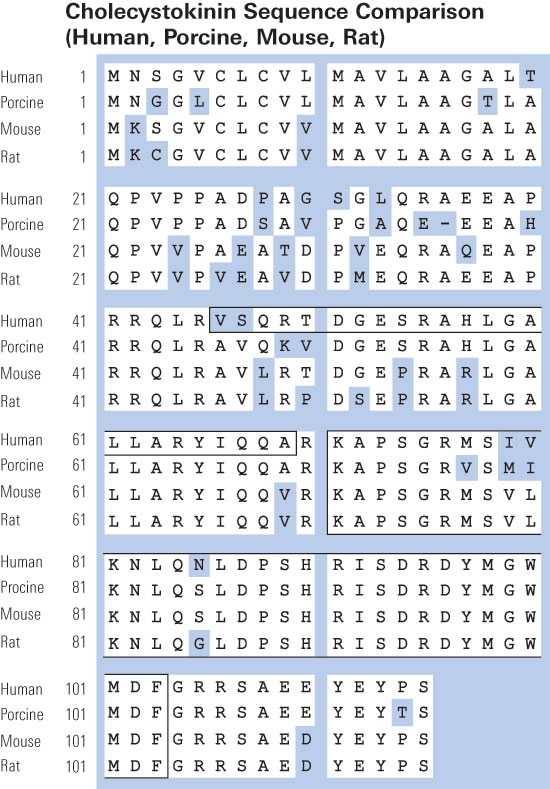 |
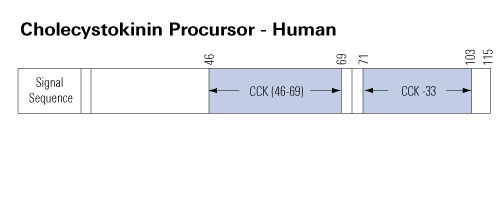
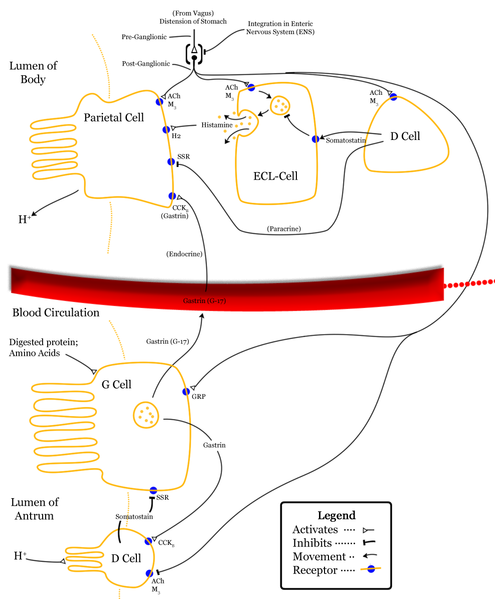
Cholecystokinin (CCK) was discovered in 1928 in jejunal extracts as a gallbladder contraction factor. It was later shown to be member of a peptide family, which are all ligands for the CCK1 and CCK2 receptors. CCK peptides are known to be synthetized in small intestinal endocrine I-cells and cerebral neurons. But in addition, CCK is expressed in several endocrine glands (pituitary cells, thyroid C-cells, pancreatic islets, the adrenals, and the testes); in peripheral nerves; in cortical and medullary kidney cells; in cardial myocytes; and in cells of the immune system. CCK peptides stimulate pancreatic enzyme secretion and growth, gallbladder contraction, and gut motility, satiety and inhibit acid secretion from the stomach. Moreover, they are major neurotransmitters in the brain and the periphery. CCK peptides also stimulate calcitonin, insulin, and glucagon secretion, and they may act as natriuretic peptides in the kidneys. CCK peptides are derived from proCCK with a C-terminal bioactive YMGWMDFamide sequence, in which the Y-residue is partly O-sulfated. The plasma forms are CCK-58, -33, -22, and -8, whereas the small CCK-8 and -5 are potent neurotransmitters. Over the last decades, CCK expression has also been encountered in tumors (neuroendocrine tumors, cerebral astrocytomas, gliomas, acoustic neuromas, and specific pediatric tumors). Recently, a metastastic islet cell tumor was found to cause a specific CCKoma syndrome, suggesting that circulating CCK may be a useful tumor marker.
Rehfeld JF. Front Endocrinol (Lausanne). 2017;8:47.
Two separate experiments were performed to localize the gastrointestinal sites of action regulating meal size (MS), intermeal interval (IMI) length and satiety ratio (SR, IMI/MS) by cholecystokinin (CCK) 8 and 33. Experiment 1: CCK-8 (0, 0.05, 0.15, 0.25nmol/kg) was infused in the celiac artery (CA, supplies stomach and upper duodenum) or the cranial mesenteric artery (CMA, supplies small and part of the large intestine) prior to the onset of the dark cycle in free feeding, male Sprague Dawley rats and MS (normal rat chow), IMI and SR were recorded. Experiment 2: CCK-33 (0, 0.05, 0.15, 0.25nmol/kg) were infused in the CA or the CMA, under the same experimental conditions above, and MS, IMI and SR were recorded. Experiment 1 found that CCK-8 reduces MS, prolongs the IMI and increases the SR at sites supplied by both arteries. Experiment 2 found that CCK-33 reduces MS and increases the SR at sites supplied by the CMA. We conclude that in male rats the feeding behaviors evoked by CCK-33, but not CCK-8, are regulated at specific gastrointestinal sites of action.
Washington MC, Mhalhal TR, Sayegh AI. Horm Behav. 2016;85:36-42.
Cholecystokinin (CCK) is a classic gut hormone. CCK is also a complex system of peptides expressed in several molecular forms in enteroendocrine I cells, in cerebral and peripheral neurons, in cardiac myocytes and spermatozoa. CCK gene expression has now been found at protein or peptide level in different neuroendocrine tumors; cerebral gliomas and astrocytomas and specific pediatric tumors. Tumor hypersecretion of CCK was recently reported in a patient with a metastatic islet cell tumor and hypercholecystokininemia resulting in a novel tumor syndrome, the cholecystokininoma syndrome. This review presents an overview of the cell-specific biogenesis of CCK peptides, and a description of the CCK expression in tumors and of the cholecystokininoma syndrome. Finally, assays for the diagnosis of CCK-producing tumors are reviewed.
Rehfeld JF. Cholecystokinin expression in tumors: biogenetic and diagnostic implications. Future Oncol. 2016;12(18):2135-47.
We investigated the changes in the capacity for synthesis of the exocrine pancreas of piglets during the 2 wk after weaning at 7 d of age (trial 1) by measuring the expression of digestive enzymes at mRNA and activity levels in pancreas homogenates, and the effects of high and low feed intakes during the 1st wk postweaning (trial 2) on these measures. The trypsin mRNA level was transiently decreased 43% 3 d postweaning (P < 0.05). Thereafter, trypsin and lipase mRNAs linearly increased (P < 0.05). During the 1st wk postweaning, trypsin- and lipase-specific activities were reduced 44 and 79% (P < 0.05), respectively, whereas 14 d after weaning, trypsin was at the preweaning value and lipase was at a low level. Amylase-specific activity did not change with weaning. Plasma cholecystokinin (CCK) and gastrin concentrations decreased 1 d postweaning and increased afterward up to 3 and 5 d postweaning, respectively. By 3 d after weaning, the mRNA level of trypsin was twofold higher (P < 0.05) in piglets that consumed more feed than in those that consumed less, whereas 7 d after weaning, the groups did not differ. By 7 d after weaning, the specific activity of amylase was higher, and lipase-specific activity was lower, in piglets that consumed more feed than in those that consumed less. Plasma CCK and gastrin concentrations measured 7 d after weaning were correlated with feed intake (r = +0.56 and r = +0.68, P < 0.05, respectively). In conclusion, by 3 d postweaning, pancreatic exocrine function was adapting to the new diet. Afterward, the expression of specific genes coding digestive enzymes and the levels of pancreatic enzyme activities were restored or stimulated, except for lipase-specific activity. Therefore, the pancreas can adjust to weaning and dry food intake as early as wk 2 of life.
Marion J et al. J. Nutr 2003; 133:362-368
Type A CCK receptor (CCKAR) antagonists differing in blood-brain barrier permeability [devazepide penetrates; the dicyclohexylammonium alf of Na-3-quinolinoyl-D-Glu-N,N-dipentylaminde (A-70104) does not] were used to test the hypothesis that duodenal nutrient-induced inhibition of gastric emptying is mediated by CCKARs located peripheral to the blood-brain barrier. Rats received A-70104 (700 or 3,000 nmol . kg-1. h-1) or devazepide (2.5 umol/kg iv) and either a 15-min intravenous infusion of CCK-8 (3nmol.kg-1.h-1) or duodenal infusion of casein, peptone, Intralipid, or maltose. Gastric emptying of saline was measured during the last 5 min of each infusion. A-70104 and devazepide abolished the gastric emptying response to a madximal inhibitory dose of CCK-8. Each of the macronutrients inhibited gastric emptying. A-70104 and devazepide attenuated inhibitory responses to each macronutrient. Intravenous injection of a CCK antibody to immunoneutralize circulating CCK had no effect on peptone or Intralipid-induced responses. Thus endogenous CCK appears to act in part by a paracrine or neurocrine mechanism at CCKARs peripheral to the blood-brain barrier to inhibit gastric emptying.
Reidelberger et al. Am J Physiol Regul Integr Comp Physiol 2003; 284: R66-R75
The presence of nutrients in the small intestine slows gastric emptying and suppresses appetite and food intake; these effects are partly mediated by the release of gut hormones, including CCK. We investigated the hypothesis that the modulation of antropyloroduodenal motility, suppression of appetite, and stimulation of CCK and glucagon-like peptide-1 secretion by intraduodenal fat are dependent on triglyceride hydrolysis by lipase. Sixteen healthy, young, lean men were studied twice in double-blind, randomized, crossover fashion. Ratings for appetite-related sensations, antropyloroduodenal motility, and plasma CCK and glucagon-like peptide-1 concentrations were measured during a 120-min duodenal infusion of a triglyceride emulsion (2.8 kcal/min) on one day with, on the other day without, 120 mg tetrahydrolipstatin, a potent lipase inhibitor. Immediately after the duodenal fat infusion, food intake at a buffet lunch was quantified. Lipase inhibition with tetrahydrolipstatin was associated with reductions itonic and phasic pyloric pressures, increased numbers of isolated antral and duodenal pressure waves, and stimulation of antropyloroduodenal pressure-wave sequences (all P < 0.05). Scores for prospective consumption and food intake at lunch were greater, and nausea scores were slightly less, and the rises in plasma CCK and glucagon-like peptide-1 were abolished (all P < 0.05). In conclusion, lipase inhibition attenuates the effects of duodenal fat on antropyloroduodenal motility, appetite, and CCK and glucagon-like peptide-1 secretion.
Feinle C et al. Am J Physiol Gastrointest Liver Physiol 284: G798-G807, 2003
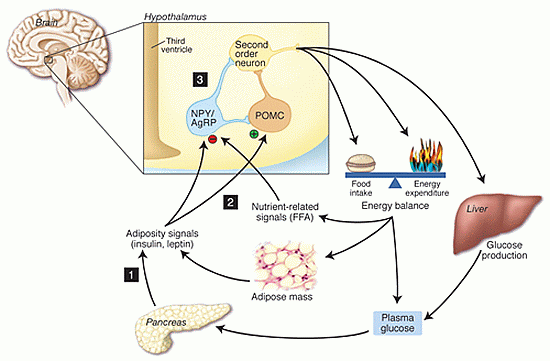
In the present study, we tested the hypothesis that a single daily injection of the gut peptide CCK, together with continuous leptin infusion, would produce significantly greater loss of body weight than leptin alone. We found that a single daily intraperitoneal injection of CCK-8 (0.5 microg/kg) significantly enhanced the weight-reducing effects of 0.5 microg/day leptin infused continuously into the lateral ventricle of male Sprague-Dawley rats by osmotic minipump. However, CCK and leptin together did not enhance reduction of daily chow intake. Furthermore, there was no synergistic reduction of 30-min sucrose intake, although a significant main effect of both leptin and CCK was observed on sucrose intake. These results 1) confirm our previous reports of synergy between leptin and CCK on body weight, 2) demonstrate that enhancement of leptin-induced weight loss does not require bolus administration of leptin, and 3) suggest that enhanced body weight loss following leptin and CCK does not require synergistic reduction of food intake by leptin and CCK.
Matson et al. Am J Physiol Regul Integr Comp Physiol 2002 May;282(5):R1368-73
The effects of intracerebroventricular administration of devezapide, a CCK(1) receptor antagonist, was investigated on food intake in rats. In the first experiment, rats (n=5) were deprived of food for 17 h and injected intracerebroventricularly with either vehicle or devazepide (1, 10, 25 or 100 ng). Five minutes after vehicle or drug administration, the animals were presented with food and intake measured for 60 min. Devazepide produced a dose-related increase in food intake. Doses of 1, 10 and 25 ng significantly increased consumption (at least P<0.01 in each case). A second experiment was subsequently undertaken to investigate whether systemic administration of the intracerebroventricular doses used in the first experiment would affect food intake. Rats (n=8) that have been deprived of food for 17 h were injected intraperitoneally with either vehicle or devazepide (3, 30, 75 or 300 ng/kg). Five minutes after vehicle or drug administration, the animals were presented with food and intake was measured for 60 min. Devazepide (3-300 ng/kg, i.p.) had no significant effects on food consumption. The results show that central administration of low doses of devazepide increase food intake in rats, while similar doses, given systemically, do not affect consumption. These findings suggest the possibility that endogenous cholecystokinin (CCK), acting at central CCK(1) receptors, may play a physiological role in the control of feeding behaviour in the rat.Ebenezer IS. Eur J Pharmacol 2002 Apr 19;441(1-2):79-82

Social Network Confirmation