
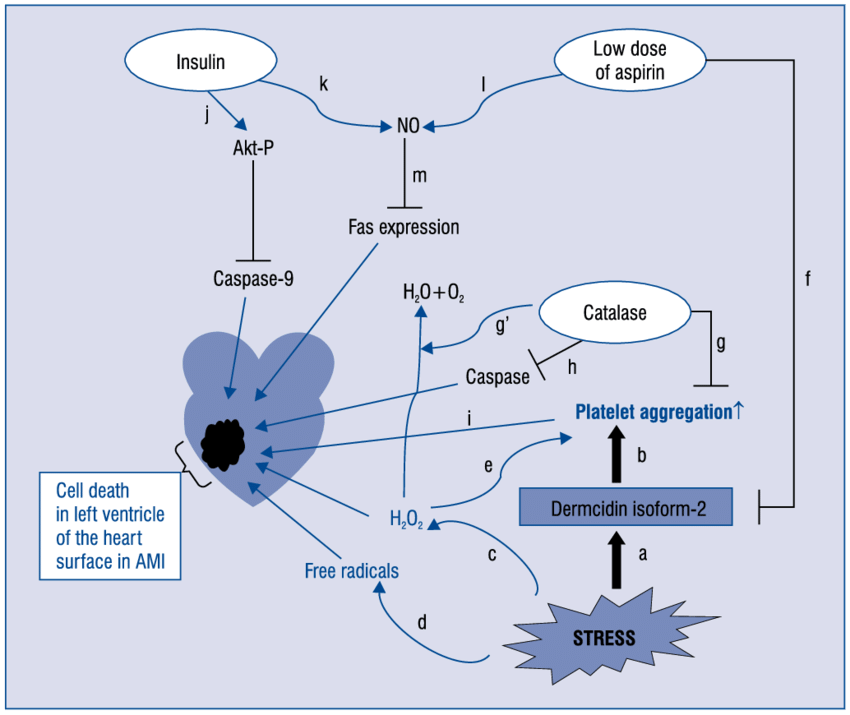
Diabetes is now epidemic worldwide. Several hundred-million peoples are presently suffering from this disease with other secondary-disorders. Stress, hypertension, sedentary life-style, carbohydrate/lipid metabolic-disorders due to genetic or environmental factors attributes to type-1 and/or type-2 diabetes. Present investigation demonstrates that stress-induced protein dermcidin isoform-2 (DCN-2) which appears in the serum of diabetic-patients play a key-role in this disease pathogenesis/severity. DCN-2 suppresses insulin production-release from liver/pancreas. It also increases the insulin-resistance. Stress-induction at the onset/progression of this disease is noticed as the high-level of lipid peroxides/low-level of free-thiols in association with increase of inflammatory-markers c-reactive protein and TNF-?. DCN-2 induced decrease in the synthesis of glucose-activated nitric oxide synthase (GANOS) and lower production of NO in liver has been shown here where NO is demonstrated to lower the expression of glucose trabsporter-4 (GLUT-4) and its translocation on liver membrane surface. This finally impairs glucose transport to organs from the extracellular fluid. Low level of glucose uptake further decreases glucose-induced insulin synthesis. The central role of DCN-2 has been demonstrated in type-1/type-2 diabetic individuals, in rodent hepatocytes and pancreatic-cell, tissue-slices, in-vitro and in-vivo experimental model. It can be concluded that stress-induced decrease in insulin synthesis/function, glucose transport is an interactive consequence of oxidative threats and inflammatory events.
Bhattacharya S, Khan MM, Ghosh C, Bank S, Maiti S. The role of Dermcidin isoform-2 in the occurrence and severity of Diabetes. Sci Rep. 2017;7(1):8252.
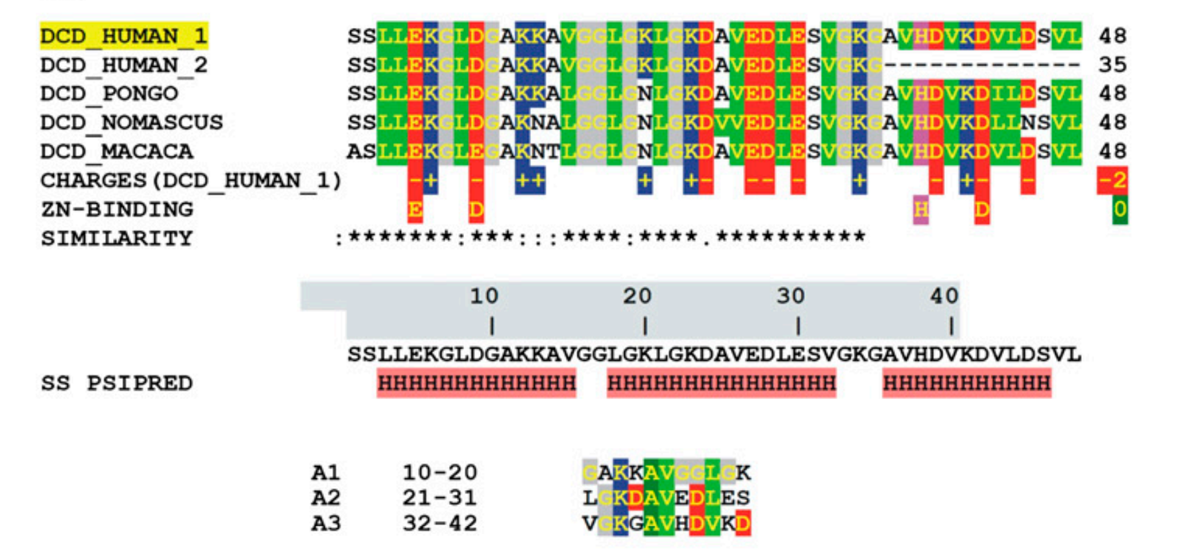
SSL-25 (SSLLEKGLDGAKKAVGGLGKLGKDA) is one of the shortest peptides present in human sweat and is produced after the proteolytic processing of the parent peptide dermcidin. Both peptides are reported to have antimicrobial function. To determine the structure of SSL-25 in lipid bilayers, a series of 19F-labeled SSL-25 analogs were synthesized. Circular dichroism (CD) analysis showed that SSL-25 and all of its analogs formed α-helices in the presence of lipid vesicles, thus allowing a detailed analysis via oriented CD and solid-state NMR. The results suggest that SSL-25 resides on the membrane surface with a slight helix tilt angle. A detailed 19F NMR analysis revealed that SSL-25 does not form a continuous helix. The ?-helical structure of the N-terminal part of the peptide was preserved in membranes of different lipid compositions and at various peptide-to-lipid molar ratios, but the C-terminus was disordered and did not fold into a well-defined α-helical conformation. Furthermore, the NMR results showed that SSL-25 resides on the membrane surface and does not re-orient into the membrane in response to changes in either peptide concentration or membrane composition. SSL-25 does not aggregate and remains fully mobile within the membrane bilayer, as shown by 19F NMR. SSL-25 has a high binding affinity toward bilayers mimicking bacterial lipid compositions, but does not bind to mammalian model membranes containing cholesterol. These observations may explain the specificity of this peptide for bacterial membranes, and they are also in line with basic biophysical considerations on spontaneous lipid curvature and the general effect of cholesterol on peptide/lipid interactions.
Mühlhäuser P, Wadhwani P, Strandberg E, Bürck J, Ulrich AS. Structure analysis of the membrane-bound dermcidin-derived peptide SSL-25 from human sweat. Biochim Biophys Acta. 2017;1859(12):2308-2318.
BACKGROUND: We previously identified dermicidin (DCD), which encodes a growth and survival factor, as a gene amplified and overexpressed in a subset of breast tumors. Patients with DCD-positive breast cancer have worse prognostic features. We therefore searched for specific molecular signatures in DCD-positive breast carcinomas from patients and representative cell lines.
METHODS: DCD expression was evaluated by qRT-PCR, immunohistochemical and immunoblot assays in normal and neoplastic tissues and cell lines. To investigate the role of DCD in breast tumorigenesis, we analyzed the consequences of its downregulation in human breast cancer cell lines using three specific shRNA lentiviral vectors. Genes up- and down-regulated by DCD were identified using Affymetrix microarray and analyzed by MetaCore Platform.
RESULTS: We identified DCD splice variant (DCD-SV) that is co-expressed with DCD in primary invasive breast carcinomas and in other tissue types and cell lines. DCD expression in breast tumors from patients with clinical follow up data correlated with high histological grade, HER2 amplification and luminal subtype. We found that loss of DCD expression led to reduced cell proliferation, resistance to apoptosis, and suppressed tumorigenesis in immunodeficient mice. Network analysis of gene expression data revealed perturbed ERBB signaling following DCD shRNA expression including changes in the expression of ERBB receptors and their ligands.
CONCLUSIONS: These findings imply that DCD promotes breast tumorigenesis via modulation of ERBB signaling pathways. As ERBB signaling is also important for neural survival, HER2+ breast tumors may highjack DCD's neural survival-promoting functions to promote tumorigenesis.
Bancovik J, Moreira DF, Carrasco D, et al. Dermcidin exerts its oncogenic effects in breast cancer via modulation of ERBB signaling. BMC Cancer. 2015;15:70.
The effect of dermcidin isoform 2 (dermcidin), an environmentally induced stress protein, was investigated on the genesis of diabetes mellitus and hypertension, the two major atherosclerotic risk factors. The role of dermcidin as an atherosclerotic risk factor related to the impaired systemic insulin level was investigated. Dermcidin was prepared by electrophoresis using plasma from the subjects with acute ischemic heart disease. Injection of 0.2μM dermcidin in mice increased the blood glucose level from 98 ± 2.45 mg/dL to 350 ± 10.2 mg/dL which was normalized by the oral administration of acetyl salicylic acid (aspirin) after 24 h. Hypertensive subjects with systolic and diastolic blood pressure of 165 mm and 95 mm of Hg, respectively, had plasma dermcidin level of 95 nM. Ingestion of acetyl salicylic acid (aspirin) (150 mg/70 kg body weight) decreased the systolic and diastolic pressures to 125 mm and 80 mm of Hg, respectively, with decrease of dermcidinlevel to 15 nM. Incubation of kidney cortex cells with 0.2 μM dermcidin-inhibited synthesis of (r)-cortexin, an antihypertensive protein, and the basal (r)-cortexin level was reduced from 33 nM to 15 nM. Addition of 25??units of insulin/mL was found to reverse the inhibition of cortexin synthesis. The effect of dermcidin as a diabetogenic and a hypertensive agent could be controlled either by aspirin or by insulin.
Ghosh R, Bank S, Bhattacharya R, Khan NN, Sinha AK. Neutralization by insulin of the hypertensive effect of dermcidin isoform 2: an environmentally induced diabetogenic and hypertensive protein. Cardiol Res Pract. 2014;2014:412815.
Y-P30, the 30 amino acid N-terminal peptide of the dermcidin gene, has been found to promote neuronal survival and differentiation. Its early presence in development and import to the fetal brain led to the hypothesis that Y-P30 has an influence on proliferation, differentiation and migration. Neurospheres derived from neural stem cells isolated from E13 mouse cortex and striatal ganglionic eminences were treated with Y-P30, however, the proportion of progenitors, neurons and astrocytes generated in differentiation assays was not altered. A short Y-P30 treatment of undifferentiated striatal and cortical neurospheres failed to alter the proportion of BrdU-positive cells. A longer treatment reduced the percentage of BrdU-positive cells and GABA-immunoreactive neurons only in striatal spheres. The presence of Y-P30 enhanced migration of T24 human bladder carcinoma cells in a wound-healing assay in vitro. Further, Y-P30 enhanced migration of T24 cells, rat primary cortical astrocytes and PC12 cells in chemotactic Boyden chamber assays. Together, these findings suggest that a major function of Y-P30 is to promote migration of neural and non-neural cell types. Dash-wagh S, Neumann JR, Veitinger S, et al. Mol Cell Neurosci. 2011;48(3):195-204.
BACKGROUND: The skin has evolved an epithelial defence mechanism which is characterized by antimicrobial peptides that inactivate various microorganisms and exhibit stimulatory activities bridging innate and adaptive immunity. Dermcidin (DCD) is a newly isolated antimicrobial peptide produced by the eccrine sweat glands in the skin. Recently, the DCD peptides DCD-1 and DCD-1L have been shown to display in vitro microbicidal activities against bacteria and viruses. OBJECTIVES: Because some skin-derived antimicrobial peptides activate keratinocytes, we investigated whether DCD-1L would also trigger keratinocyte activation. METHODS: Normal human keratinocytes were used in this study. The ability of DCD-1L to induce the production of cytokines/chemokines by keratinocytes was determined by enzyme-linked immunosorbent assay, and various inhibitors were used to investigate the stimulatory mechanism of DCD-1L. Mitogen-activated protein kinase (MAPK) phosphorylation and NF-kappaB activation were analysed by Western blotting. RESULTS: DCD-1L stimulated keratinocytes to generate cytokines and chemokines including tumour necrosis factor-alpha, interleukin-8 (CXCL8), interferon-inducible protein 10 (CXCL10) and macrophage inflammatory protein-3alpha (CCL20). To determine the molecular mechanism involved, we showed that DCD-1L-mediated cytokine/chemokine production was controlled by both G-protein and MAPK pathways, as evidenced by the inhibitory effects of pertussis toxin and specific inhibitors for p38 and ERK, but not for JNK, on DCD-1L-induced keratinocyte activation. Furthermore, we confirmed that DCD-1L could induce phosphorylation of p38 and ERK, and noticeably upregulated NF-kappaB activation. CONCLUSIONS: Taken together, the new activity of DCD-1L to stimulate the production of cytokines/chemokines by keratinocytes provides novel evidence for the implication of DCD, beyond its microbicidal ability, in skin immunity.
Niyonsaba F, et al., Br J Dermatol. 2009 Feb;160(2):243-9. Epub 2008 Nov 11.
Dermcidin (DCD) is an antimicrobial peptide which is constitutively expressed in eccrine sweat glands. By postsecretory proteolytic processing in sweat, the DCD protein gives rise to anionic and cationic DCD peptides with a broad spectrum of antimicrobial activity. Many antimicrobial peptides induce membrane permeabilization as part of their killing mechanism, which is accompanied by a loss of the bacterial membrane potential. In this study we show that there is a time-dependent bactericidal activity of anionic and cationic DCD-derived peptides which is followed by bacterial membrane depolarization. However, DCD-derived peptides do not induce pore formation in the membranes of gram-negative and gram-positive bacteria. This is in contrast to the mode of action of the cathelicidin LL-37. Interestingly, LL-37 as well as DCD-derived peptides inhibit bacterial macromolecular synthesis, especially RNA and protein synthesis, without binding to microbial DNA or RNA. Binding studies with components of the cell envelope of gram-positive and gram-negative bacteria and with model membranes indicated that DCD-derived peptides bind to the bacterial envelope but show only a weak binding to lipopolysaccharide (LPS) from gram-negative bacteria or to peptidoglycan, lipoteichoic acid, and wall teichoic acid, isolated from Staphylococcus aureus. In contrast, LL-37 binds strongly in a dose-dependent fashion to these components. Altogether, these data indicate that the mode of action of DCD-derived peptides is different from that of the cathelicidin LL-37 and that components of the bacterial cell envelope play a role in the antimicrobial activity of DCD.
Senyürek I, Paulmann M, Sinnberg T, et al. Dermcidin-derived peptides show a different mode of action than the cathelicidin LL-37 against Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53(6):2499-509.
Antimicrobial peptides from human skin are an important component of the innate immune response and play a key role as a first line of defense against infections. One such peptide is the recently discovered dermcidin-1L. To better understand its mechanism and to further investigate its antimicrobial spectrum, recombinant dermcidin-1L was expressed in Escherichia coli as a fusion protein and purified by affinity chromatography. The fusion protein was cleaved by factor Xa protease to produce recombinant dermcidin-1L. Antimicrobial and hemolytic assays demonstrated that dermcidin-1L displayed microbicidal activity against several opportunistic nosocomial pathogens, but no hemolytic activity against human erythrocytes even at concentrations up to 100 microM. Structural studies performed by circular dichroism spectroscopy indicated that the secondary structure of dermcidin-1L was very flexible, and both alpha-helix and beta-sheet structures might be required for the antimicrobial activity. Our results confirmed previous findings indicating that dermcidin-1L could have promising therapeutic potentials and shed new light on the structure-function relationship of dermcidin-1L.
Lai YP, Peng YF, Zuo Y, et al. Functional and structural characterization of recombinant dermcidin-1L, a human antimicrobial peptide. Biochem Biophys Res Commun. 2005;328(1):243-50.
In this report, we describe the identification of a polypeptide survival-promoting factor that is produced by maternal and early postnatal peripheral blood mononuclear cells (PBMCs) of the immune system in Long-Evans rats and humans. The factor, termed Y-P30, most likely arises from proteolytic processing of a larger precursor protein and accumulates mainly in pyramidal neurons of the developing cortex and hippocampus but not in astrocytes. It was released from neurons grown in culture and substantially promotes survival of cells in explant monocultures of perinatal thalamus from the offspring. Y-P30 mRNA was not detectable in infant or adult brain and was present only in blood cells of pregnant rats and humans but not in nonpregnant controls. However, Y-P30 transcription could be induced in PBMCs of adult animals by a central nervous system lesion (i.e., optic nerve crush), which points to a potential role of the factor not only in neuronal development but also in neuroinflammation after white matter injury.
Landgraf P, et al., FASEB J. 2005 Feb;19(2):225-7. Epub 2004 Dec 6.
| Catalog# | Product | Standard Size | Price |
|---|---|---|---|
| 075-33 | DCD-1L / Dermcidin (63-110) (Human) | 100 µg | $317 |
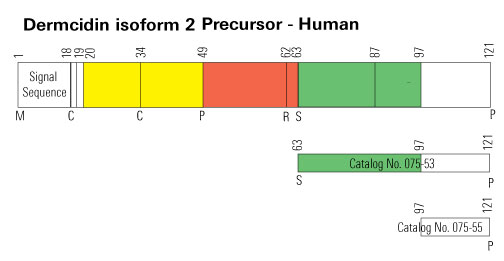
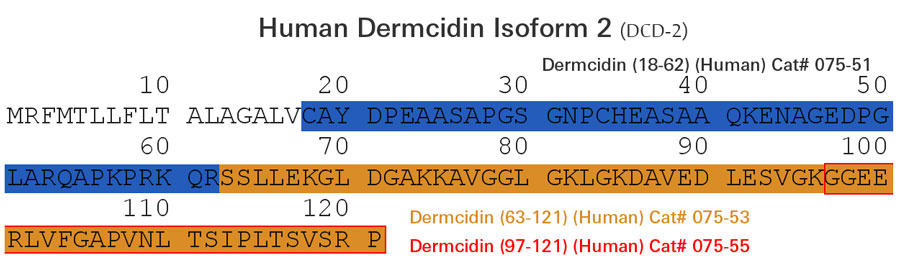
| 075-51 | Dermcidin (18-62) (Human) | 100 µg | $231 |
| 075-52 | Dermcidin (20-62) (Human) | 100 µg | $226 |
| 075-57 | Dermcidin (63-87) / SSL-25 (Human) | 100 µg | $186 |
| 075-53 | Dermcidin Isoform-2 (63-121) (Human) | 100 µg | $371 |
| 075-55 | Dermcidin Isoform-2 (97-121) (Human) | 100 µg | $186 |
| 075-32 | YP-30 / Dermcidin (20-49) (Human) | 100 µg | $152 |
Social Network Confirmation