Catalog # |
Size |
Price |
|
|---|---|---|---|
| 070-88 | 5 mg | $133 |
 )
)
|
Phe-Val-Phe-Leu-Met
|
| 635.35 | |
|
| ≥ 96% |
|
| Exhibits correct molecular weight |
|
| Insoluble in water. Dissolve in 80% acetonitrile. Then dilute with water or any desired buffer. |
|
|
Up to 6 months in lyophilized form at 0-5°C. For best results, rehydrate just before use. After rehydration, keep solution at +4°C for up to 5 days or freeze at -20°C for up to 3 months. Aliquot before freezing to avoid repeated freeze-thaw cycles. |
|
| White powder |
|
| Each vial contains 5 mg of NET peptide. |
The pharmacological modulation of collagen turnover is a strategy potentially useful in different skin conditions. The serine protease inhibitor Serpin A1 and portions of its C-terminal region have been investigated as collagen modulators. To clarify the mechanisms by which the C-terminal 409-418 peptide SA1-III (same peptide of alpha-1-Antitrypsin (385-394) / A1AT-ST1C5 10mer (Human). Phoenix cat. #066-74) increases extracellular type I collagen levels, to compare its activities range with that of the originator molecule Serpin A1, and to evaluate its efficacy in primary cultures from adult and aged human subjects. The different forms of type I collagen were analyzed by means of western blot in cell lysates and cell-conditioned media of primary human dermal fibroblasts obtained from subjects of different ages. Gelatin zymography was used to investigate the degrading enzymes. Cell viability and in vitro wound healing tests were used to evaluate cell proliferation. The SA1-III peptide increased extracellular collagen levels by reducing degradation, with no effect on cellular biosynthesis or cell proliferation mechanisms. A reduced level of MMP-2 and MMP-9 was also found in cell media upon peptide treatment. No peptide effect was detected on inflammatory mediators gene expression in resting and LPS-stimulated fibroblasts, or in the wound healing test. The SA1-III peptide is a good collagen modulator candidate, protecting collagen against degradation without detectable actions on biosynthesis, acting at reasonably low concentrations, and non-interfering with cell proliferation. It is effective in primary fibroblasts from young and aged subjects. These effects can prove useful in pathological and physiological skin conditions in which collagen degradation is excessive compared to the synthetic capacity.
Cipriani C, Pascarella S, Errante F, et al. Serpin A1 and the modulation of type I collagen turnover: Effect of the C-terminal peptide 409-418 (SA1-III) in human dermal fibroblasts. Cell Biol Int. 2018;42(10):1340-1348.
Human SERPINA1 gene is located on chromosome 14q31-32.3 and is organized into three (IA, IB, and IC) non-coding and four (II, III, IV, V) coding exons. This gene produces α1-antitrypsin (A1AT), a prototypical member of the serpin superfamily of proteins. We demonstrate that human peripheral blood leukocytes express not only a product corresponding to the transcript coding for the full-length A1AT protein but also two short transcripts (ST1C4 and ST1C5) of A1AT. In silico sequence analysis revealed that the last exon of the short transcripts contains an Open Reading Frame (ORF) and thus putatively can produce peptides. We found ST1C4 expression across different human tissues whereas ST1C5 was mainly restricted to leukocytes, specifically neutrophils. A high up-regulation (10-fold) of short transcripts was observed in isolated human blood neutrophils after activation with lipopolysaccharide. Parallel analyses by liquid chromatography-mass spectrometry identified peptides corresponding to C-terminal region of A1AT in supernatants of activated but not naïve neutrophils. Herein we report for the first time a tissue specific expression and regulation of short transcripts of SERPINA1 gene, and the presence of C-terminal peptides in supernatants from activated neutrophils, in vitro. This gives a novel insight into the studies on the transcription of SERPINA1 gene.
Matamala N, Aggarwal N, Iadarola P, et al. Identification of Novel Short C-Terminal Transcripts of Human SERPINA1 Gene. PLoS ONE. 2017;12(1):e0170533.
Human SERPINA1 gene is located on chromosome 14q31-32.3 and is organized into three (IA, IB, and IC) non-coding and four (II, III, IV, V) coding exons. This gene produces ?1-antitrypsin (A1AT), a prototypical member of the serpin superfamily of proteins. We demonstrate that human peripheral blood leukocytes express not only a product corresponding to the transcript coding for the full-length A1AT protein but also two short transcripts (ST1C4 and ST1C5) of A1AT. In silico sequence analysis revealed that the last exon of the short transcripts contains an Open Reading Frame (ORF) and thus putatively can produce peptides. We found ST1C4 expression across different human tissues whereas ST1C5 was mainly restricted to leukocytes, specifically neutrophils. A high up-regulation (10-fold) of short transcripts was observed in isolated human blood neutrophils after activation with lipopolysaccharide. Parallel analyses by liquid chromatography-mass spectrometry identified peptides corresponding to C-terminal region of A1AT in supernatants of activated but not naïve neutrophils. Herein we report for the first time a tissue specific expression and regulation of short transcripts of SERPINA1 gene, and the presence of C-terminal peptides in supernatants from activated neutrophils, in vitro. This gives a novel insight into the studies on the transcription of SERPINA1 gene.
Matamala N, Aggarwal N, Iadarola P, et al. PLoS ONE. 2017;12(1):e0170533.
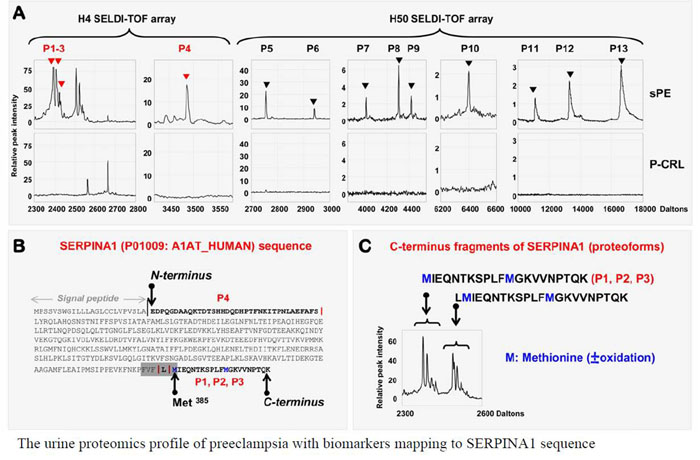
Preeclampsia, a pregnancy-specific disorder, shares typical pathophysiological features with protein misfolding disorders including Alzheimer's disease. Characteristic for preeclampsia is the involvement of multiple proteins of which fragments of SERPINA1 and β-amyloid co-aggregate in urine and placenta of preeclamptic women. To explore the biophysical basis of this interaction, we investigated the multidimensional efficacy of the FVFLM sequence in SERPINA1, as a model inhibitory agent of β-amyloid aggregation. After studying the oligomerization of FVFLM peptides using all-atom molecular dynamics simulations with the GROMOS43a1 force field and explicit water, we report that FVFLM can aggregate and its aggregation is spontaneous with a remarkably faster rate than that recorded for KLVFF (aggregation "hot-spot" from β-amyloid). The fast kinetics of FVFLM aggregation was found to be driven primarily by core-like aromatic interactions originating from the anti-parallel orientation of complementarily uncharged strands. The conspicuously stable aggregation mechanism observed for FVFLM peptides is found not to conform to the popular 'dock-lock' scheme. We also found high propensity of FVFLM for KLVFF binding. When present, FVFLM disrupts the β-amyloid aggregation pathway and we propose that FVFLM-like peptides might be used to prevent the assembly of full-length Aβ or other pro-amyloidogenic peptides into amyloid fibrils.
Kouza M, Banerji A, Kolinski A, Buhimschi IA, Kloczkowski A. Phys Chem Chem Phys. 2017;19(4):2990-2999.
MALDI-TOF spectrometry has not been used for urinary exosome analysis. We used it for determining UC biomarkers. From 2012 to 2015, we enrolled 129 consecutive patients with UC and 62 participants without UC. Exosomes from their urine were isolated, and analyzed through MALDI-TOF spectrometry. Immunohistochemical (IHC) analysis of another 122 UC and 26 non-UC tissues was conducted to verify the discovered biomarkers. Two peaks at m/z 5593 (fragmented peptide of alpha-1-antitrypsin; sensitivity, 50.4%; specificity, 96.9%) and m/z 5947 (fragmented peptide of histone H2B1K sensitivity, 62.0%; specificity, 92.3%) were identified as UC diagnosis exosome biomarkers. UC patients with detectable histone H2B1K showed 2.29- and 3.11-fold increased risks of recurrence and progression, respectively, compared with those with nondetectable histone H2B1K. Verification results of IHC staining revealed significantly higher expression of alpha 1-antitrypsin (p?=?0.038) and H2B1K (p?=?0.005) in UC tissues than in normal tissues. The expression of alpha 1-antitrypsin and H2B1K in UC tissues was significantly correlated with UC grades (p < 0.05). Urinary exosome proteins alpha 1-antitrypsin and histone H2B1K, which are identified through MALDI-TOF analysis, could facilitate rapid diagnosis and prognosis of UC.
Lin SY, Chang CH, Wu HC, et al. Sci Rep. 2016;6:34446.
Systemic inflammatory response syndrome (SIRS) is a life threatening condition and the leading cause of death in intensive care units. Although single aspects of pathophysiology have been described in detail, numerous unknown mediators contribute to the progression of this complex disease. The aim of this study was to elucidate the pathophysiological role of CAAP48, a C-terminal alpha-1 antitrypsin fragment, that we found to be elevated in septic patients and to apply this peptide as diagnostic marker for infectious and noninfectious etiologies of SIRS. Incubation of human polymorphonuclear neutrophils with synthetic CAAP48, the SNP-variant CAAP47, and several control peptides revealed intense neutrophil activation, induction of neutrophil chemotaxis, reduction of neutrophil viability, and release of cytokines. We determined the abundance of CAAP48 in patients with severe sepsis, severe SIRS of noninfectious origin, and viral infection. CAAP48 levels were 3-4-fold higher in patients with sepsis compared to SIRS of noninfectious origin and allowed discrimination of those patients with high sensitivity and specificity. Our results suggest that CAAP48 is a promising discriminatory sepsis biomarker with immunomodulatory functions, particularly on human neutrophils, supporting its important role in the host response and pathophysiology of sepsis.
Blaurock N, Schmerler D, Hünniger K, et al. Mediators Inflamm. 2016;2016:6129437.
Hepatic steatosis (HS) can exacerbate acute pancreatitis (AP). This study aimed to investigate the relation between ?1-antitrypsin (AAT) and acute pancreatitis when patients have HS. Using proteomic profiling, we identified 18 differently expressed proteins pots in the serum of rats with or without HS after surgical establishment of AP. AAT was found to be one of the significantly down-regulated proteins. AAT levels were significantly lower in hepatic steatosis acute pancreatitis (HSAP) than in non-HSAP (NHSAP) (P < 0.001). To explore the clinical significance of these observations, we measured the levels of AAT in the serum of 240 patients with HSAP, NHSAP, fatty liver disease (FLD), or no disease. Compared with healthy controls, serum AAT levels in patients with NHSAP were significantly higher (P < 0.01), while in patients with HSAP serum AAT levels were significantly lower (P < 0.01). Further studies showed that acute physiology and chronic health evaluation (APACHE-II) scores were negatively correlated with serum AAT levels (r = -0.85, P < 0.01). In conclusion, low serum levels of AAT in patients with HSAP are correlated with disease severity and AAT may represent a potential target for therapies aiming to improve pancreatitis.
Wang Q, Du J, Yu P, et al. Sci Rep. 2015;5:17833.
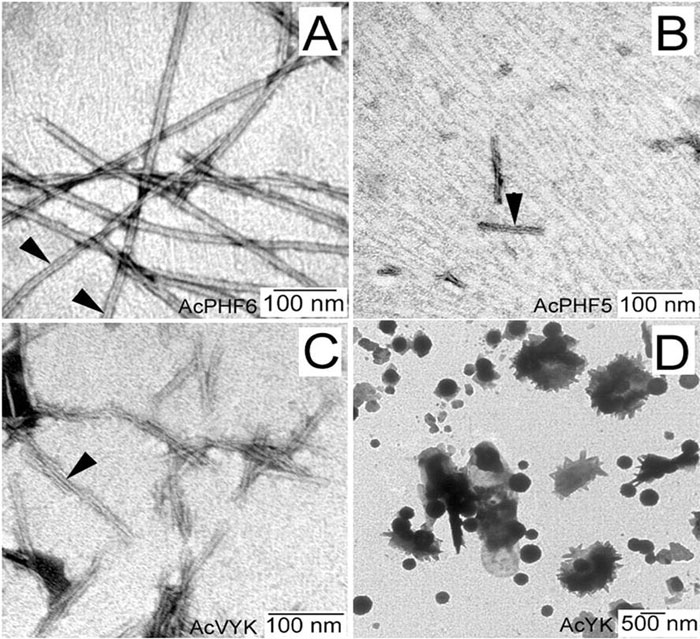
Tau protein was scanned for highly amyloidogenic sequences in amphiphilic motifs (X)(n)Z, Z(X)(n)Z (n ? 2), or (XZ)(n) (n ? 2), where X is a hydrophobic residue and Z is a charged or polar residue. N-Acetyl peptides homologous to these sequences were used to study aggregation. Transmission electron microscopy (TEM) showed seven peptides, in addition to well-known primary nucleating sequences Ac(275)VQIINK (AcPHF6*) and Ac(306)VQIVYK (AcPHF6), formed fibers, tubes, ribbons, or rolled sheets. Of the peptides shown by TEM to form amyloid, Ac(10)VME, AcPHF6*, Ac(375)KLTFR, and Ac(393)VYK were found to enhance the fraction of ?-structure of AcPHF6 formed at equilibrium, and Ac(375)KLTFR was found to inhibit AcPHF6 and AcPHF6* aggregation kinetics in a dose-dependent manner, consistent with its participation in a hybrid steric zipper model. Single site mutants were generated which transformed predicted amyloidogenic sequences in tau into non-amyloidogenic ones. A M11K mutant had fewer filaments and showed a decrease in aggregation kinetics and an increased lag time compared to wild-type tau, while a F378K mutant showed significantly more filaments. Our results infer that sequences throughout tau, in addition to PHF6 and PHF6*, can seed amyloid formation or affect aggregation kinetics or thermodynamics.
Moore CL, Huang MH, Robbennolt SA, et al. Biochemistry. 2011;50(50):10876-86.
Amyloid fibrils of diverse origin are known to disturb vital cellular functions and induce cell death. In this study, the effects of amyloid fibrils from the C-terminal fragment (C-36) of cleaved alpha1-antitrypsin (AAT) on low-density lipoprotein (LDL) metabolism were investigated in HepG2 cells. Treatment of the cells with C-36 fibrils (10 micromol/L) enhanced 125I-LDL binding and uptake 10 to 15 times, and highly up-regulated levels of LDL receptor mRNA, as compared with control cells. Competition experiments using excess of unlabeled LDL and blockage experiments with a monoclonal LDL receptor antibody diminished or completely abolished the stimulatory effects of fibrils on LDL binding and LDL receptor mRNA levels, suggesting that fibrils act via the LDL receptor pathway. However, C-36 fibrils had no significant effect on [2-14C]acetate incorporation into cholesterol biosynthesis and cholesterol ester formation, but inhibited 125I-LDL degradation by 20% and reduced bile acid biosynthesis up to 48% in a dose-dependent manner. Preincubation of the cells with fibrils before the addition of LDL totally abolished the LDL inhibitory effect on unesterified cholesterol synthesis, further confirming the LDL receptors to be the target for C-36 fibrils. Moreover, the expression of sterol regulatory element binding protein-1 (SREBP-1) was found to increase twofold and more after 24 hours of incubation of the cells with several concentrations of C-36 fibrils. Our study suggests that the cytotoxicity of C-36 fibrils on HepG2 cells is associated with perturbed intracellular cholesterol homeostasis, induced through fibril-stimulated expression of the LDL receptors via the sterol-responsive element.
Janciauskiene S, Lindgren S. Hepatology. 1999;29(2):434-42.
Fragments from various proteolytically degraded precursor proteins can form beta-amyloid fibrils. We studied, by electron microscopy and quantitative Congo red binding, the ability of three synthetic peptides, corresponding to residues 359-374 (C-36), 370-374 (C-5) and 375-394 (C-20) from the C-terminal part of alpha 1-antitrypsin (AAT) to form beta-amyloid fibrils in vitro. The peptides C-36 and C-5 had a pronounced tendency to form fibrils. C-20 lacked this property, suggesting that residues 359-375 and/or 370-374 are most critical for fibril formation. Native AAT added to peptide 125I-C-36 could bind and form complexes with the peptide, resulting in inhibition of amyloid fibril formation. Moreover, native AAT added to preformed fibrils induced disaggregation of fibrillar structures. The structural rearrangements of AAT that occurred during this 'autointeraction' included polymerization of the serpin, and an increase of its thermal stability. Also, following interaction, an increase (20-40%) of AAT's antielastase activity was noted. The demonstration of an in vitro beta-amyloid fibril formation from the AAT derived C-terminal peptides C-36 and C-5 and its regulation by the intact AAT molecule may have important in vivo implications.
Janciauskiene S, Carlemalm E, Eriksson S. Biol Chem Hoppe-Seyler. 1995;376(7):415-23.
Social Network Confirmation